
Re-envisioning the clinical research experience


With Viewpoint, researchers can more efficiently manage clinical research operations, creating better connections between study participants, sites and sponsors.
Verily Viewpoint
Optimizing operations to accelerate clinical research and trial timelines
Digital Protocols
Drive trial efficiencies with a digital study blueprint
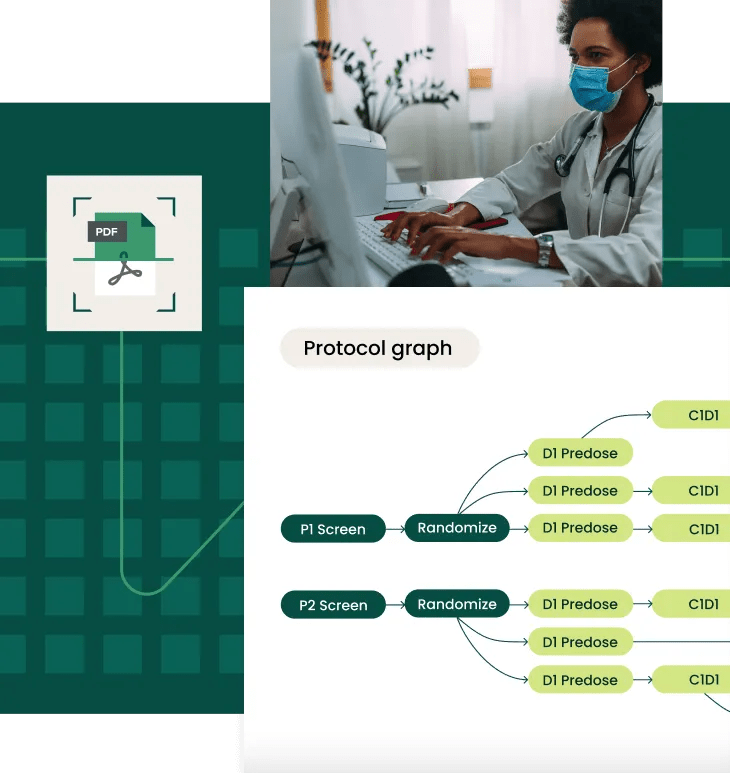
Refined over 8+ years, Verily’s proprietary, AI-driven digital protocol solution helps sponsors achieve faster, less costly trial operations by quickly capturing nuanced study details from static PDF protocols — the schedule of assessments, I/E criteria, footnotes, and more — to create a dynamic, digital data model in relevant data standards for interoperability.
Efficiency-driving benefits
Accelerate configuration of clinical operations systems, such as CTMS or electronic data capture (EDC), using a standard, machine-readable protocol
Orchestrate actions and data flow across these systems with Verily's platform — fueled by the digital model
Connect with over 450 sites using Viewpoint Site CTMS and digital protocols to speed up study startup and execution
Note: Some features are under development
Site CTMS
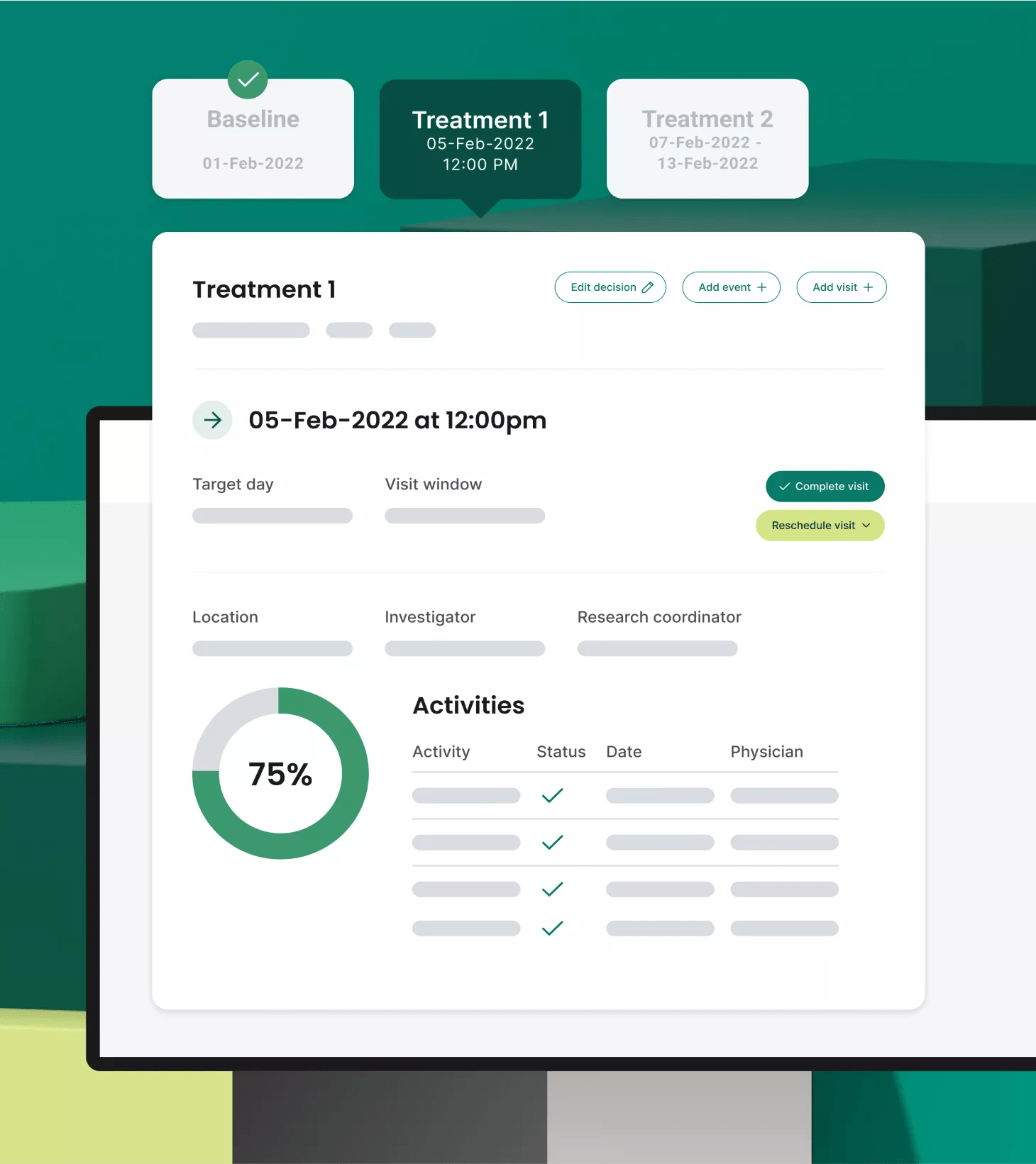
Streamline site workflows with an advanced clinical trial management system
Alleviate research gridlock for your enterprise study sites through advanced, intuitive CTMS features that support the ease, efficiency and quality of research. With Site CTMS, your organization also benefits from proprietary protocol digitization technology that configures PDF protocols into digitized workflows within the CTMS to free up your teams and power operations.