Verily Viewpoint
Accelerate clinical research
Built on Verily Pre, an AI native platform for precision health, Viewpoint solutions innovate and transform research through gathering and curating real-world data (RWD) to enable faster, more precise findings, and by providing tools for more efficient trial operations. Viewpoint connects participants, sites, and sponsors across the research lifecycle — cutting time to richer insights and breakthroughs.
Evidence Generation Solutions
Collect and integrate real-world data
Verily Viewpoint Evidence
Empower end-to-end evidence generation
Traditional RWD can lack clinical context. Viewpoint Evidence fills these gaps by connecting you to engaged, re-contactable participants and their health histories, evolving RWD from static snapshots to ongoing views of patient journeys.
- Access integrated data, unifying clinical records with survey and lifestyle data.
- Utilize clean, fit-for-purpose variables — curated from both structured and unstructured data.
- Contact participants with PROs and custom research to provide a clearer picture of patient journeys.

Drawing from Verily’s existing, engaged and consented participant base, easily re-engage individuals for streamlining new studies, deploying surveys, data linkage, and more.

Study Ops Solutions
Optimize trial operations at every stage
For study sites
Tools for study activation, clarity, control and efficiency
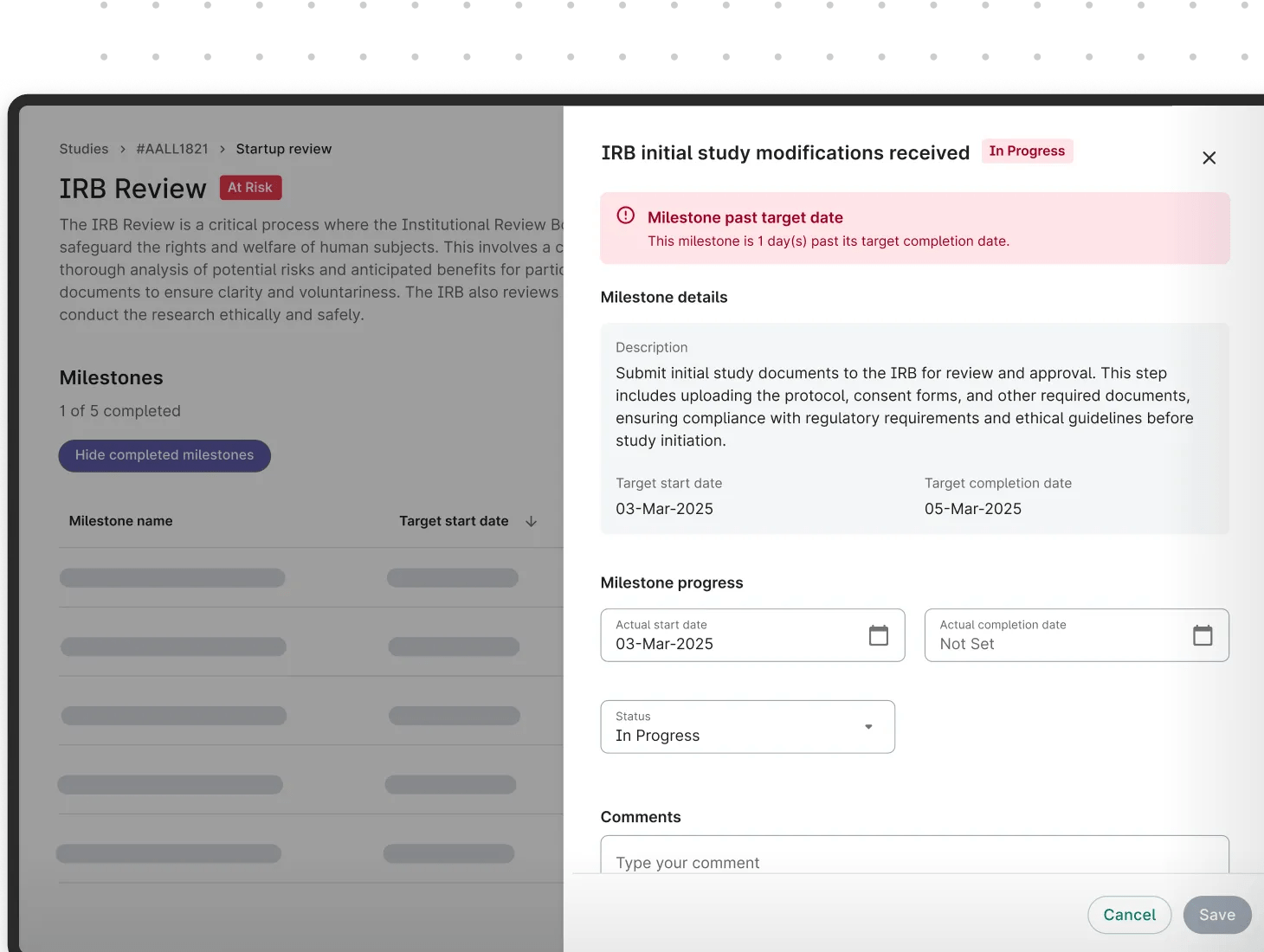
Seamlessly integrating with Site CTMS, Verily Viewpoint Study Startup helps solve ongoing challenges of delayed trial activation and enrollment, and project setbacks. Not one-site-fits-all, Study Startup offers unparalleled flexibility, allowing sites to build their own “site standard” for activation. Gain a deeper view of your activation process and promote accountability to accelerate research.
For study sites
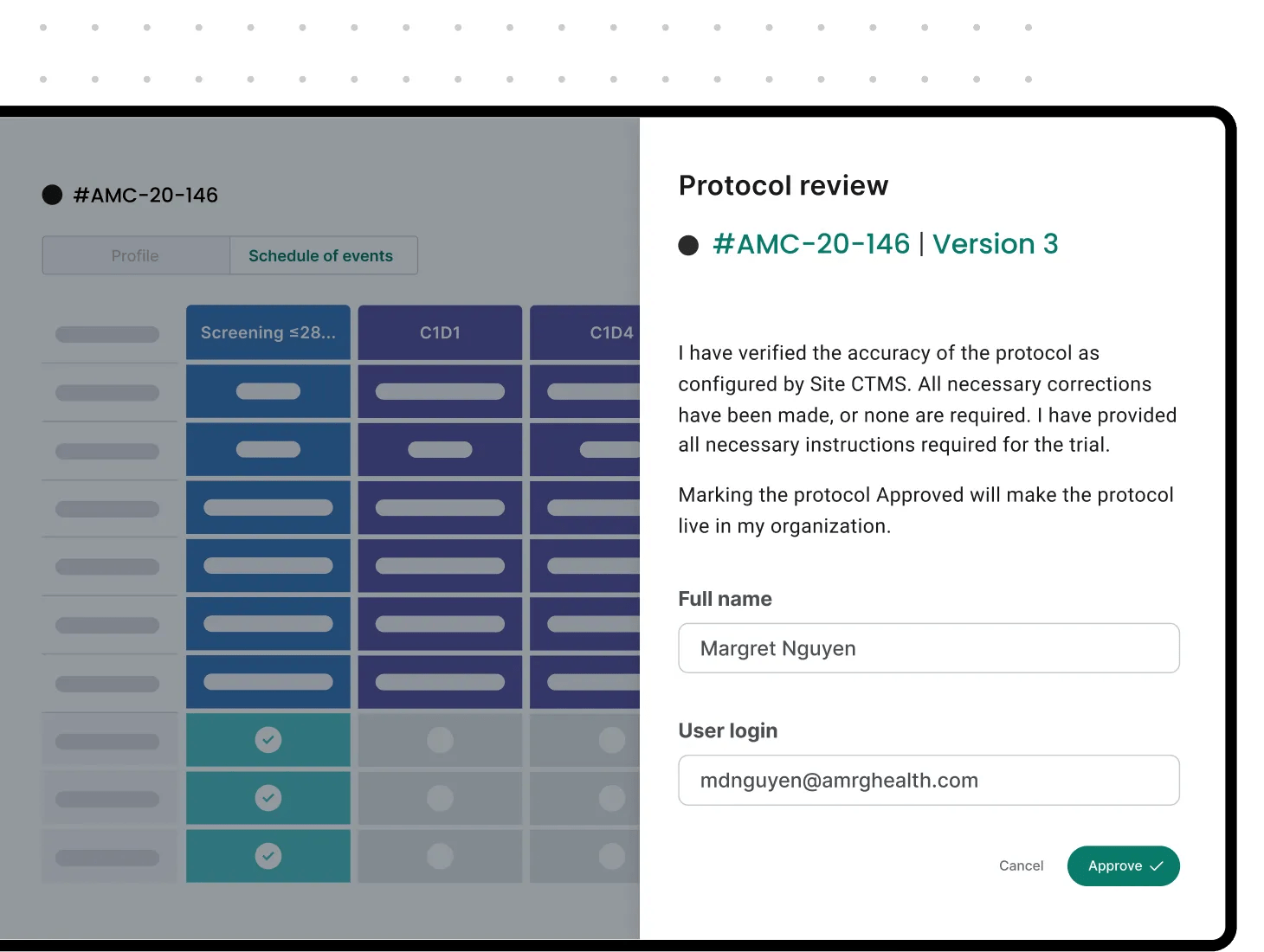
An advanced CTMS for automated, fast workflows
Alleviate research gridlock for your enterprise study sites through an intuitive clinical trial management system with features for easier, faster, higher-quality research. The Verily Viewpoint Site CTMS also offers your organization proprietary, AI-driven protocol digitization technology that configures PDF protocols into digitized workflows to free up your teams and power operations.
For study sponsors
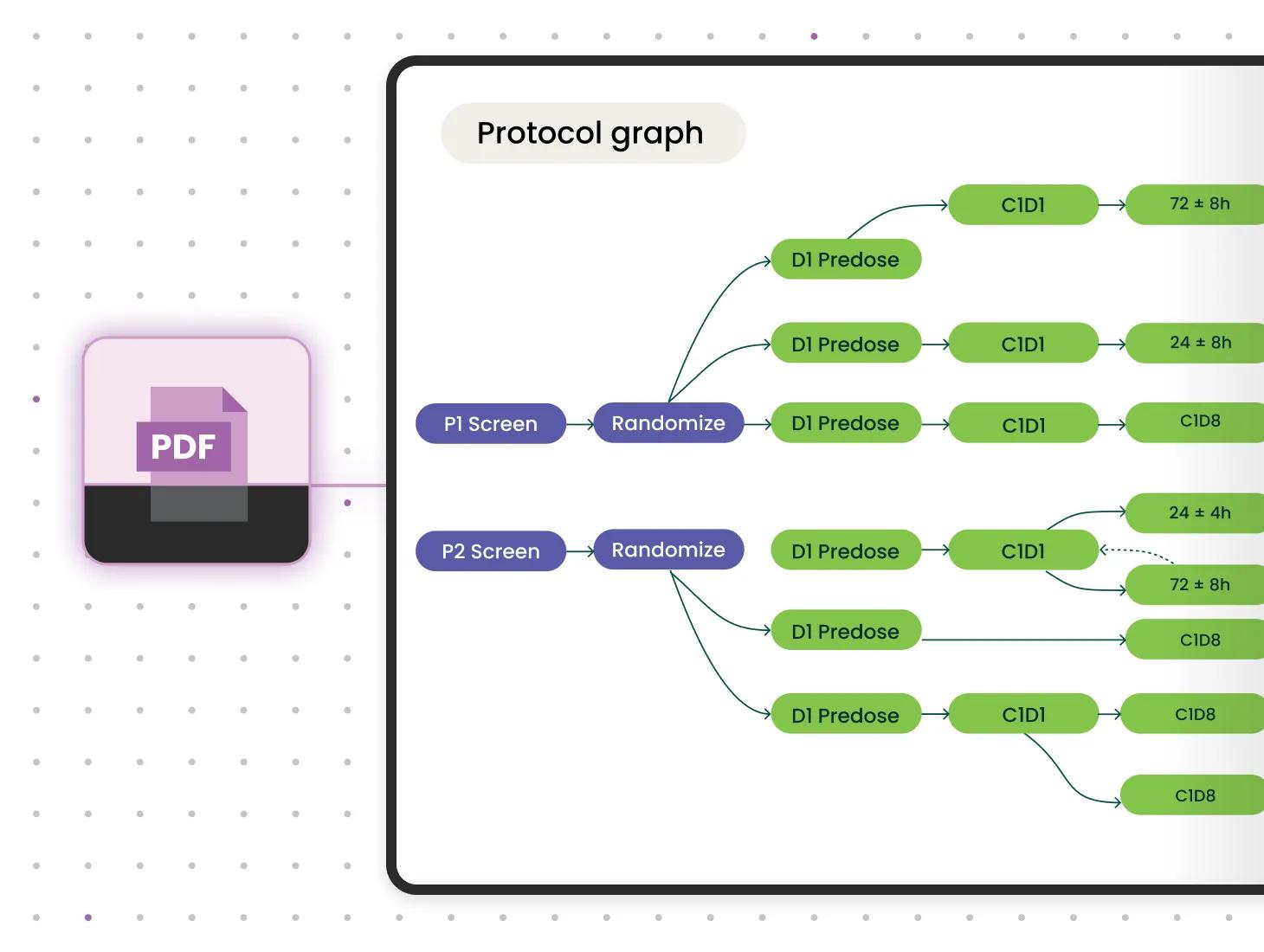
AI-driven digital protocols to save time and money
Verily Viewpoint Digital Protocols enables study sponsors to easily transition to more streamlined, digital trial operations. Run on the Verily Pre platform, AI-driven tech quickly captures all nuanced details from static PDF protocols, creating a proprietary data model. This dynamic, but standardized, digital blueprint fits within protocol authoring systems and orchestrates trial workflows.
Note: Some features are under development.