Wednesday, June 12, 2024
Proposed framework to evaluate the ability of digital measures to track early stage Parkinson’s disease progression
Generate and translate patient-centered digital measures of health into meaningful, real-world evidence for accelerating research and development through an end-to-end solution of hardware, software and machine learning.
Effective digital measures start with engaging, effective devices. Fortify research with precision measures captured from devices that fit both your study and participant needs.
With Numetric Watch*, enable non-invasive, near real-time and effective long-term collection of participant data in real-world settings for investigational use across therapeutic areas.
*Investigational use only. Exclusively for clinical investigation. Limited by Federal (or United States) law to investigational use.

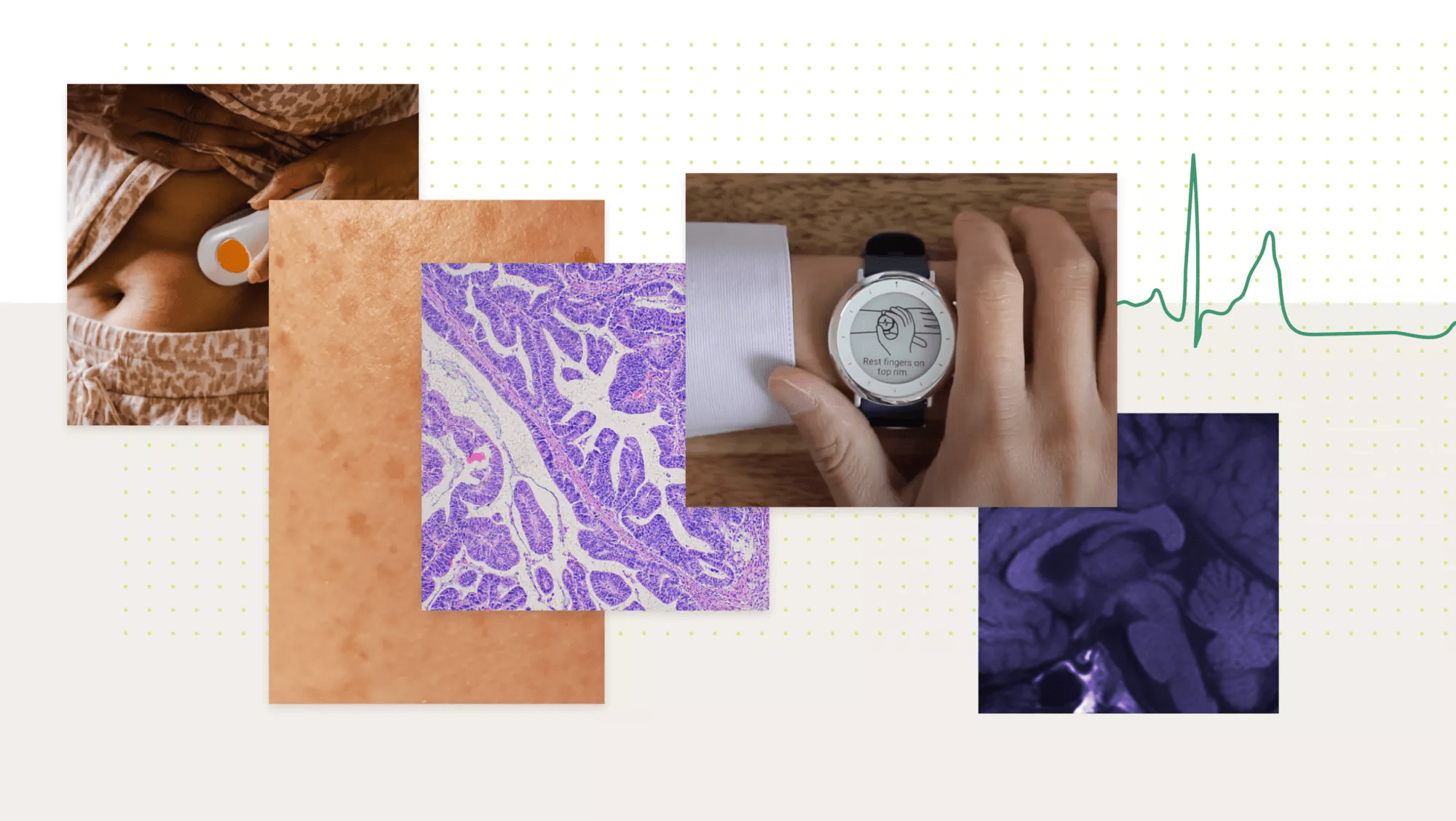
Demonstrating clinical and analytical viability of digital measures is imperative.
Through our digital biomarker platform, we help you derive fit-for-purpose, real-world evidence and study endpoints by leveraging raw data and reliable, proven algorithms to translate biometrics into digital biomarkers.

We partner with you to support novel evidence generation methods, and support your study success by helping keep a pulse on the evolving biomarker and regulatory landscape.
The 'Virtual Motor Exam’, created by Verily and enabled by their Digital Biomarkers Platform, has allowed us to carry out remote neurological exams for Parkinson’s Disease that capture objective measures with high accuracy and confidence, offering the promise of faster, more accurate research.
Bas Bloem, MD, PhD, Professor of Neurology at Radboud University Medical
Center, Nijmegen, The Netherlands
Enrich your clinical studies with raw, high-resolution and objective data that can be used and re-used for analysis and algorithms. And propel your research through development of algorithms that generate novel endpoints.

Wednesday, June 12, 2024
Proposed framework to evaluate the ability of digital measures to track early stage Parkinson’s disease progression
Wednesday, June 12, 2024
Developing composite measures that track physical activity change in people with early-stage Parkinson’s disease using machine learning and wearable sensors
Friday, May 24, 2024
Increasing psychopharmacology clinical trial success rates with digital measures and biomarkers: Future methods
Tuesday, May 7, 2024
Measuring Physical Functioning Using Wearable Sensors in Parkinson Disease and Chronic Obstructive Pulmonary Disease (the Accuracy of Digital Assessment of Performance Trial Study): Protocol for a Prospective Observational Study
Wednesday, April 3, 2024
Real-world walking behaviors are associated with early-stage heart failure: a Project Baseline Health Study
Tuesday, September 26, 2023
Validation of a Deep Learning Algorithm for Continuous, Real-Time Detection of Atrial Fibrillation Using a Wrist-Worn Device in an Ambulatory Environment
Friday, June 30, 2023
Real-life heart rate assessment: a digital biomarker for autonomic dysfunction in early Parkinson’s disease?
Friday, June 30, 2023
Real-life monitoring of Parkinson’s disease tremor: does the non-tremor subtype really exist?
Tuesday, June 13, 2023
Enhancing Participant Engagement in Clinical Studies: Strategies Applied in the Personalized Parkinson Project
Friday, April 21, 2023
Accuracy and Reliability of a Suite of Digital Measures of Walking Generated Using a Wrist-Worn Sensor in Healthy Individuals: Performance Characterization Study
Tuesday, March 14, 2023
Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson's disease in Japan
Thursday, January 5, 2023
Wrist-worn sensor-based measurements for drug effect detection with small samples in people with Lewy Body Dementia
Monday, December 26, 2022
Lingering impacts on sleep following the Daylight Savings Time transition in the Project Baseline Health Study
Friday, October 21, 2022
An Algorithm to Classify Real-World Ambulatory Status From a Wearable Device Using Multimodal and Demographically Diverse Data: Validation Study
Monday, May 23, 2022
Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function
Friday, March 19, 2021
Crowdsourcing Digital Health Measures to Predict Parkinson’s Disease Severity: The Parkinson’s Disease Digital Biomarker DREAM Challenge
Thursday, August 20, 2020
Deep Learning for Automated Sleep Staging Using Instantaneous Heart Rate
Wednesday, December 11, 2019
Quantifying Neurologic Disease Using Biosensor Measurements In-Clinic and in Free-living Settings in Multiple Sclerosis
Wednesday, July 17, 2019
The Personalized Parkinson Project: Examining Disease Progression Through Broad Biomarkers in Early Parkinson’s Disease
*For investigational use only. Exclusively for clinical investigation. Limited by Federal (or United States) law to investigational use.