Solving burnout and tech overload in clinical research

Are research sites at their breaking point?
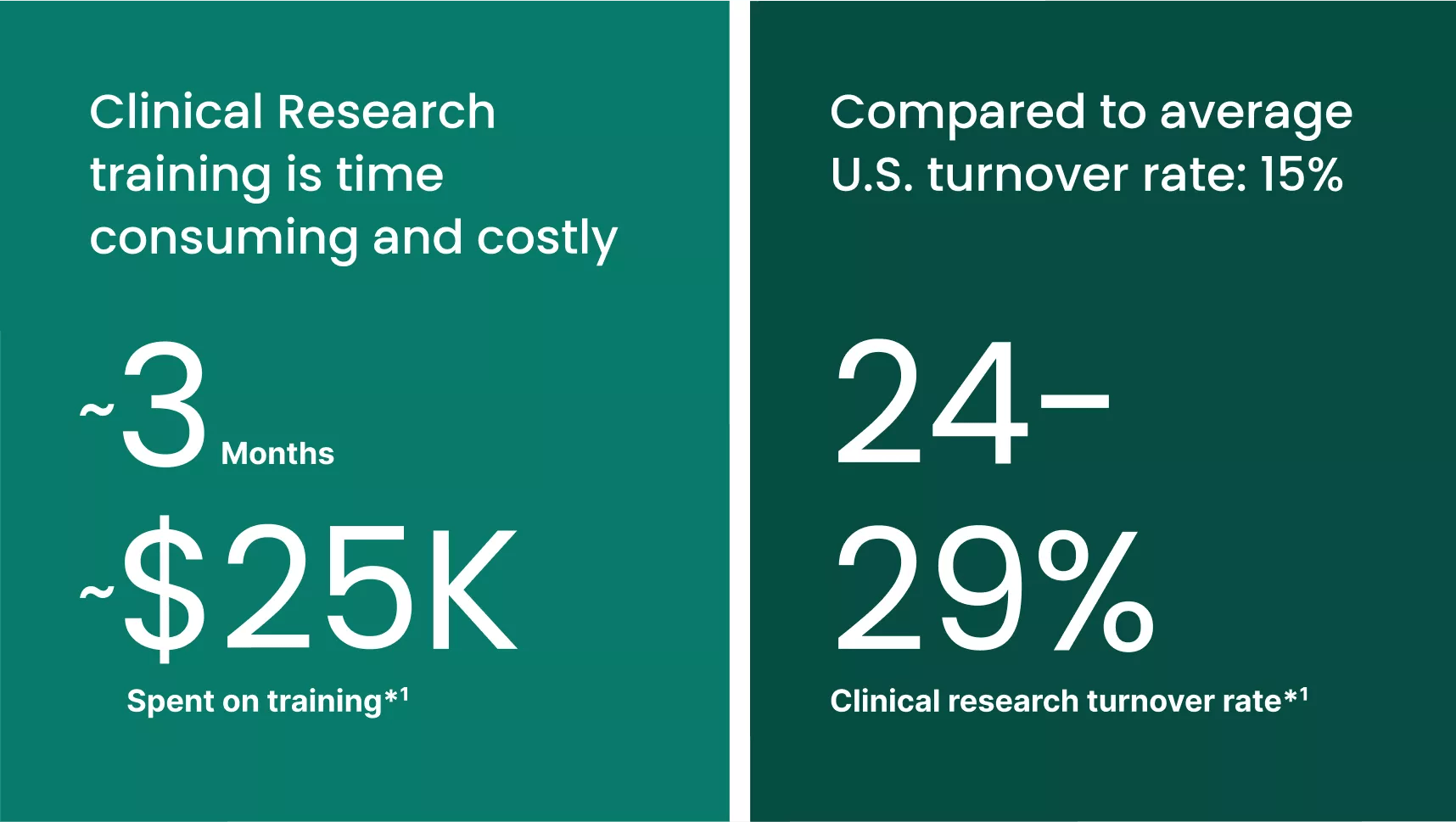
Research teams today rely on a vast electronic ecosystem: software, applications and spreadsheets all housing diverse, complex data. And yet, while having multiple tools is intended to support productivity, research staff are instead experiencing “technology overload”. It’s no wonder training a new clinical research professional can take three months and cost $25,000.*1 This learning curve is high because sites may have to log into more than six platforms for an average study.2 And this doesn’t account for the fact that teams often manage multiple studies at once!
How has the clinical research industry even got to this point? In what other profession is it routinely acceptable for employees to learn a double-digit number of systems just to get through their workday? True, the research workforce has shown remarkable adaptability, but the industry may be reaching a threshold, if not there already. The “Great Resignation” hasn’t left the clinical research industry unscathed, putting sites into a tailspin. It’s crystal clear, too many hard-to-use technologies for understaffed teams is a perfect recipe for employee turnover and burnout.
It doesn’t have to be this way. Clinical research teams can address technology overload and burnout in two ways. First, by adopting an advanced CTMS. Next, by ensuring that their CTMS is intuitive.
What is a CTMS and how can it address technology overload?
A clinical trial management system (CTMS) is a site-centric solution that can help simplify site operations from study planning to closeout.3 As a central hub, a CTMS is crucial to not only improve overall site efficiency, but to also address burnout by eliminating tedious, repetitive work that bog down staff. An advanced CTMS should:
- Digitize and organize complex workflows (so teams don’t have to)
- Eliminate redundant tasks by seamlessly integrating with other systems
- Easily visualize and track the financial health and revenue of a study
In other words, a CTMS improves research site operations, workflows and task hand offs by acting as a single source of truth across research, finance and leadership teams.
How can a user-friendly CTMS improve burnout in clinical research?
Software alone isn’t enough to truly combat burnout in clinical research. The user-friendliness of health technologies – or rather, the lack thereof – can worsen staff burnout and turnover within clinical research, and endangers site productivity.
To understand the importance of easy-to-use technologies, consider these risks without it:
Quality and safety
Studies suggest that “clinicians who work in multiple care settings using disparate technologies may struggle with the differences in interface design, with adverse impact on patient safety.”4 In a world where research teams are working in many different systems, sub-par design can impede accurate data collection, adversely impact the precision of research and ultimately, expose individuals to unnecessary risk.
Cost containment
Given the proliferation of various clinical technologies, teams waste a significant portion of their valuable time on systems training when they could be focused on actual research. What is the use of buying any eClinical technology with all the bells and whistles if these features are difficult to find, sync and use?
Optimal productivity
Each minute is critical for research organizations managing weary staff and facing costly onboarding from high turnover.4 Intuitive technology can influence productivity and well-designed interfaces speed work. Conversely, poorly designed interfaces steal time from busy schedules.4
What makes a CTMS easy to use?
The pillars of user-friendly technology are effectiveness, efficiency and user satisfaction.5 That last piece – user satisfaction – is one that if overlooked in a CTMS, may literally come at a cost to research teams.
For research and finance teams who are frustrated with clunky, outdated systems, Verily Viewpoint Site CTMS (formerly SignalPath by Verily) is an intuitive solution developed for researchers, by researchers. From our Google and Alphabet heritage, we bring best-in-class user experience (UX) practices and principles to build the most easy-to-use, accessible workflows possible. Site CTMS ensures the following for your site.
Precisely the right information for each team member
Managing a clinical studies portfolio requires coordination among members of the research, finance, regulatory and leadership teams. With this in mind, Site CTMS helps teams implement intuitive workflows by serving the right information granularity to each stakeholder. Researchers can quickly forecast the daily and weekly flow of patient schedules from one screen, finance can easily audit a study’s running accounts payable – and accounts receivable – in one viewpoint. Lastly, leadership can clearly visualize and track specific study and full portfolio statuses in one place.
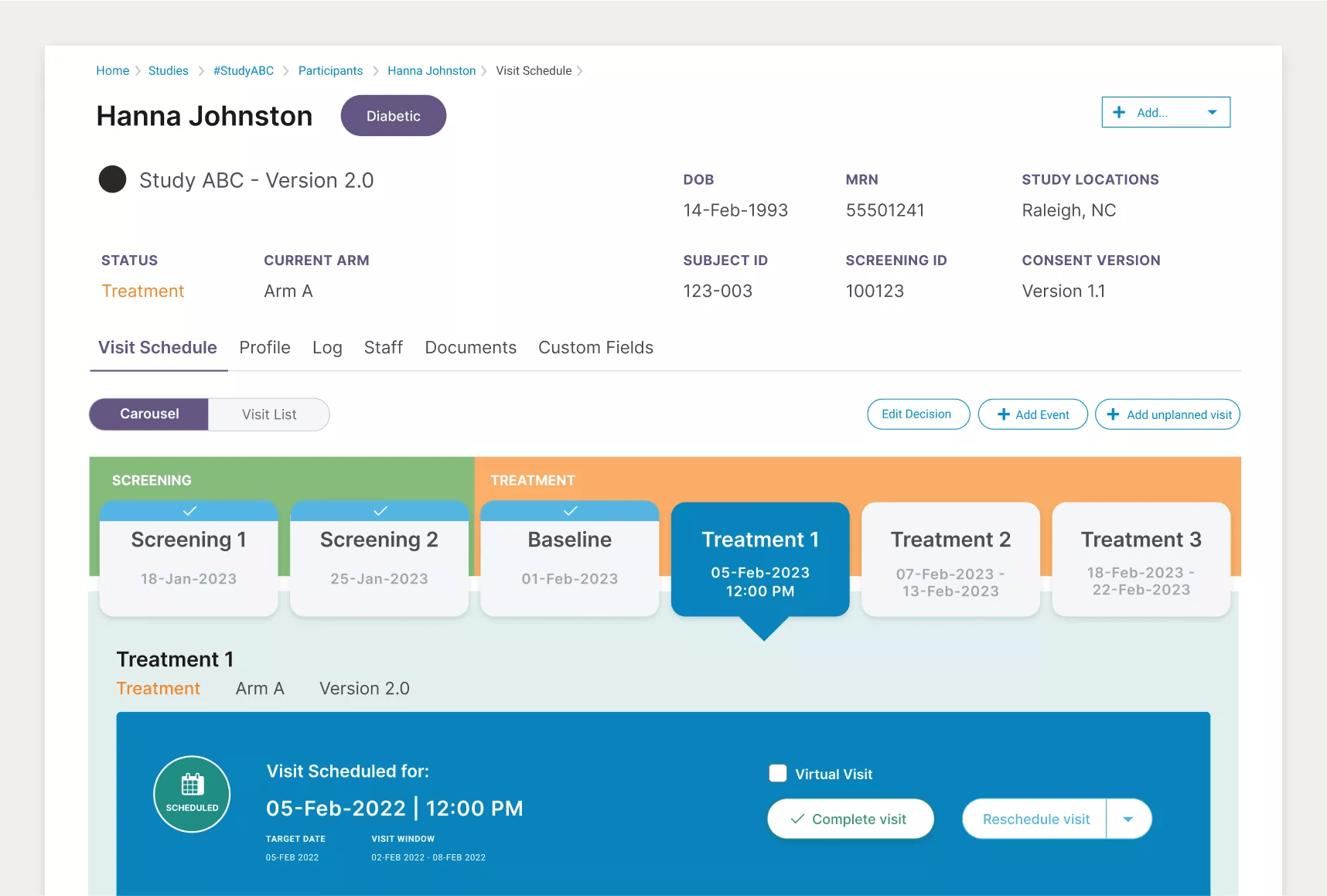
Key information within a click or two
Trying to remember where links are located, like under which tab, and playing a game of tag to navigate to the right screen is a huge waste of time. An easy-to-use CTMS should surface all key information within a few clicks of the current screen. Site CTMS does just that.
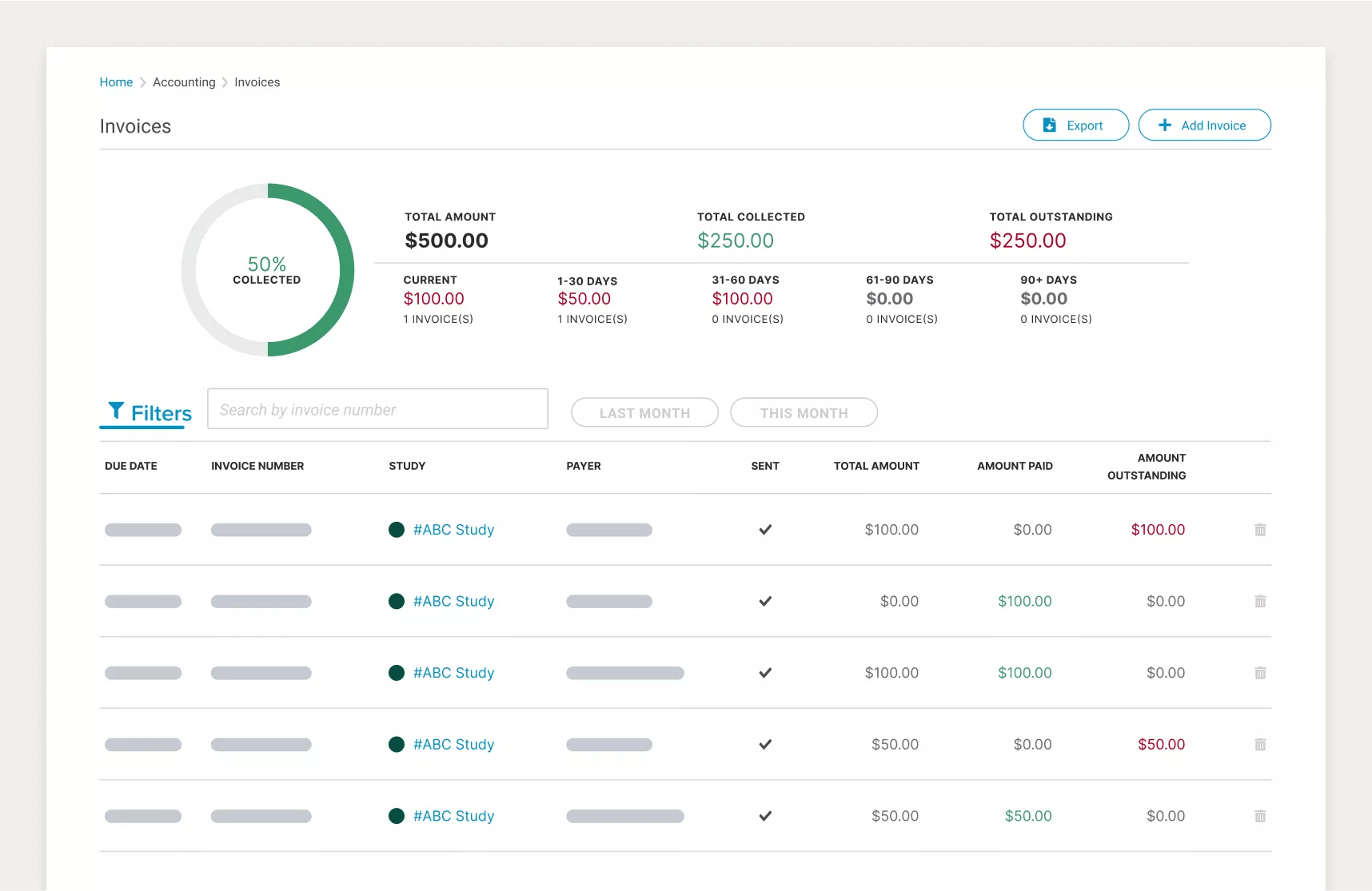
In the example below, Site CTMS allows a financial analyst to reconcile accounts by navigating from a top line, consolidated view, to all the recently entered receivables in just a click. With one more, they see exactly which study and participant the receivables came from.
Minimal context shifting
Given the numerous technologies sites use to manage their studies, reviewing clinical trial documents can be painful, especially when reconciling different versions across disparate systems. With Site CTMS, intuitively-designed interfaces help reduce “context shifting” by serving as a single repository for documents and contacts to be easily shared and communicated.
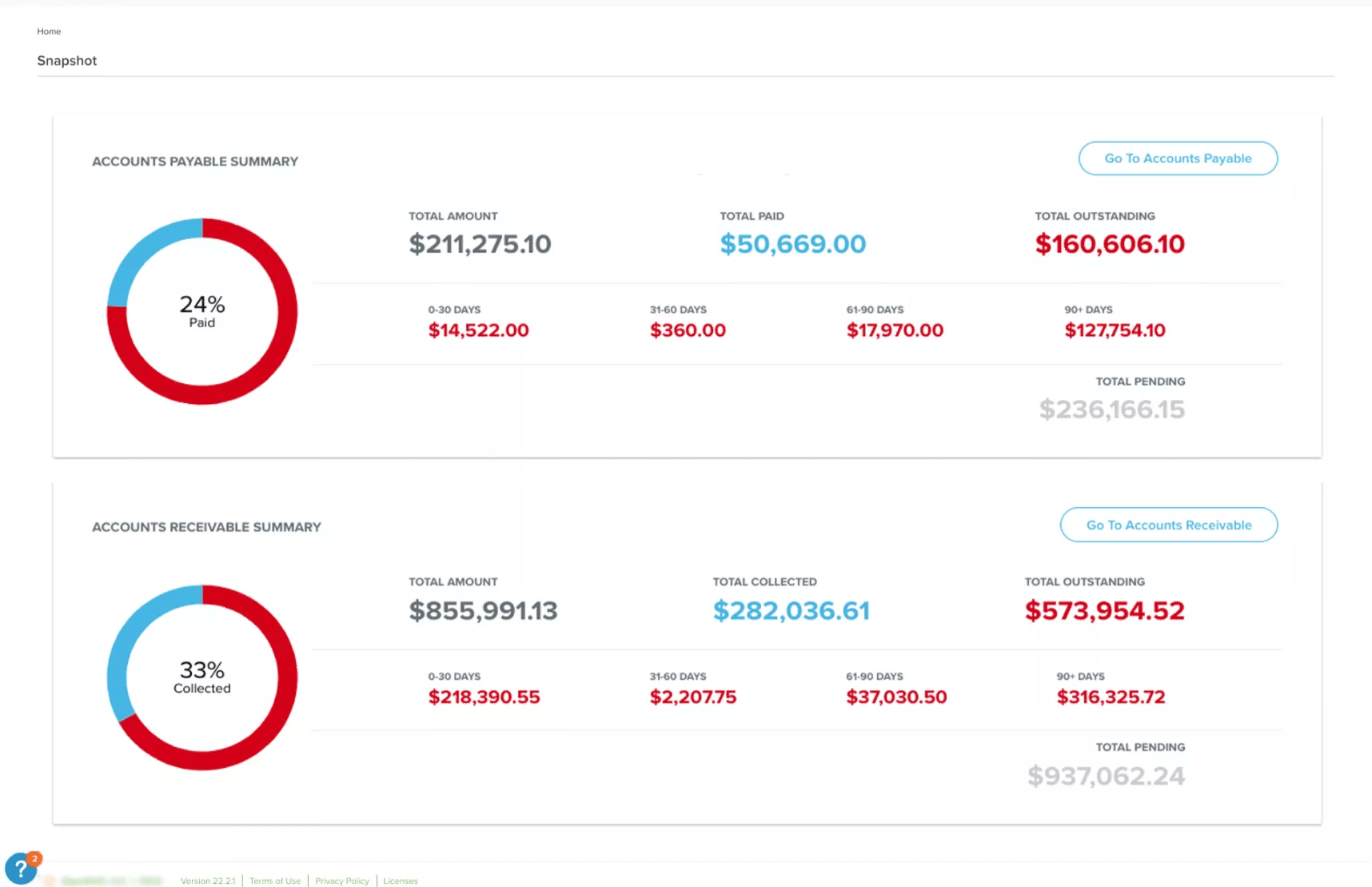
Demonstrated below, Site CTMS houses all study documents and contacts. Users can send files with comments to anybody within the platform. This feature also ensures data security, circumventing third-party emailing systems that may violate data policies.
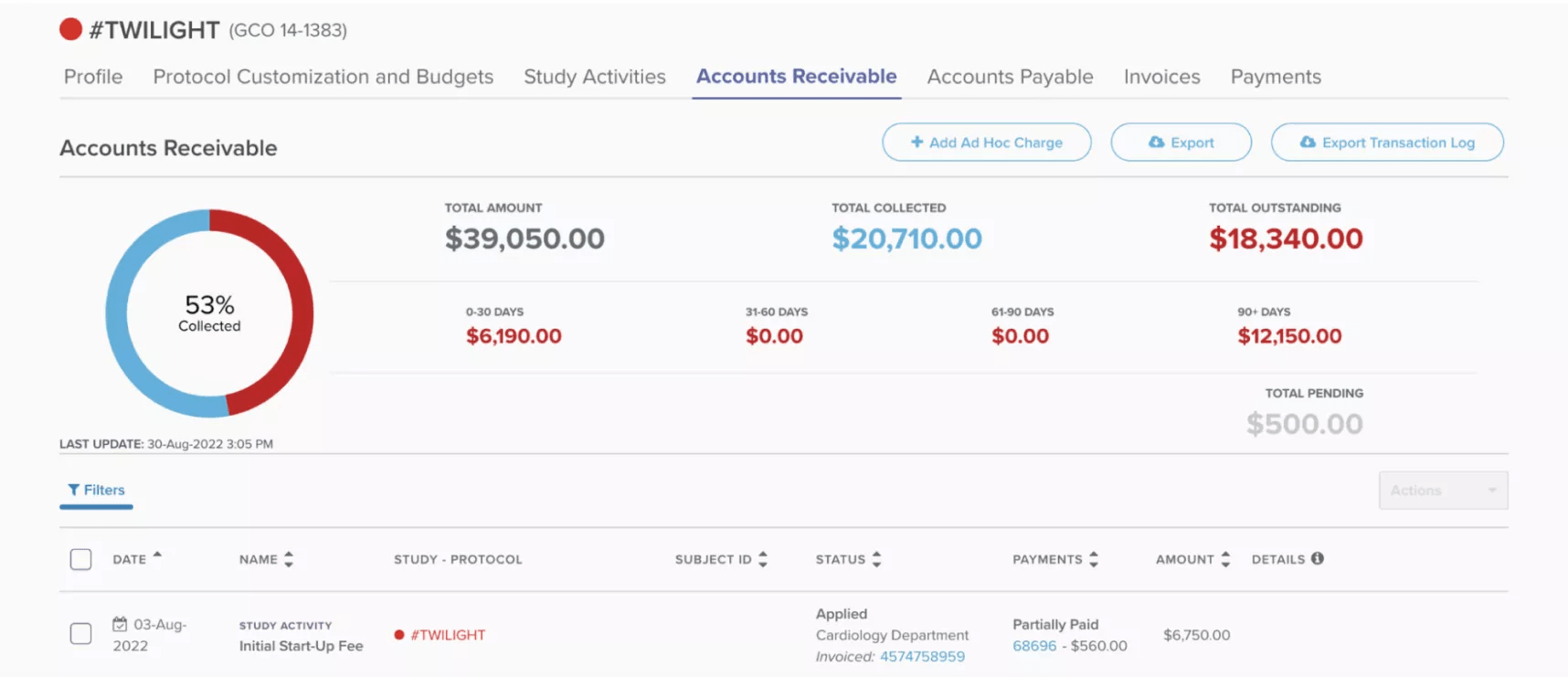
Key financial information at the study level in a single screen
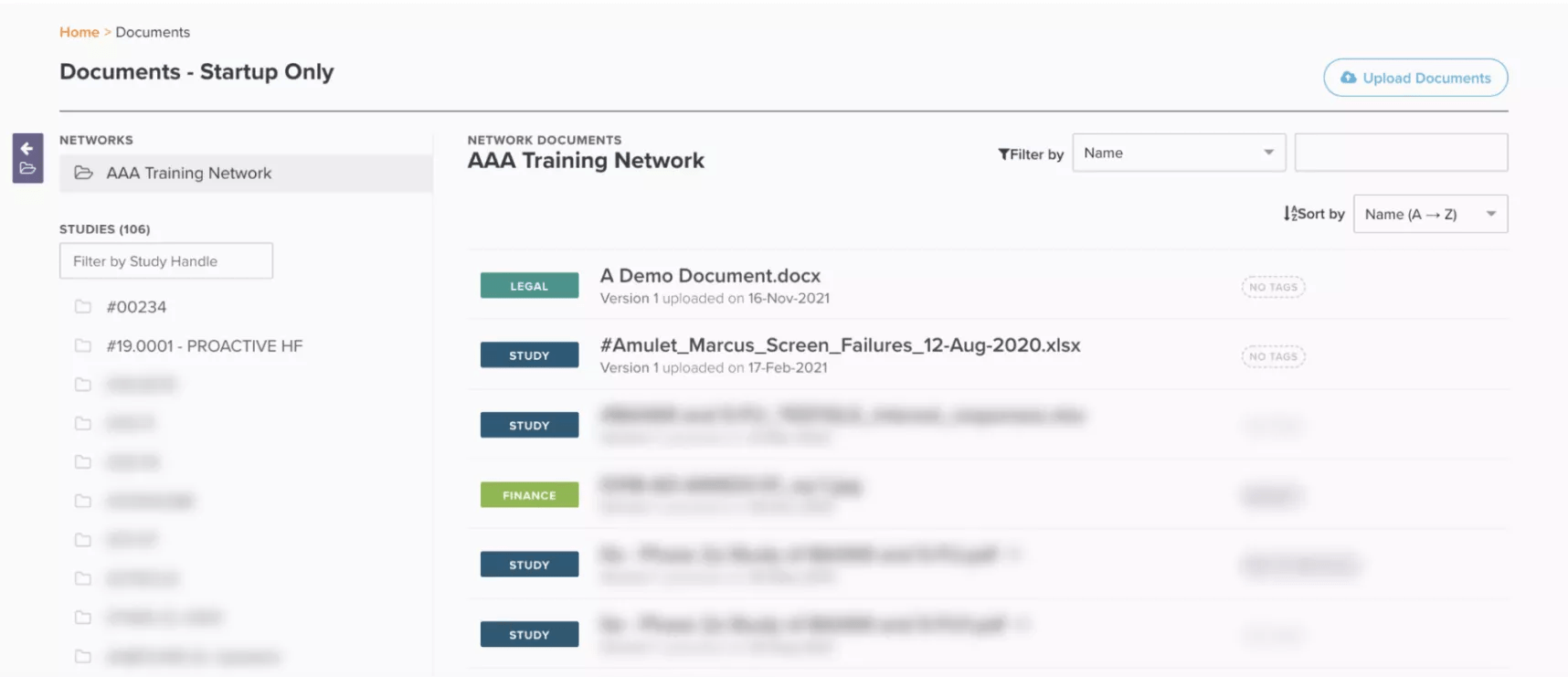
Filter and view documents all in one place
Easily search a single directory of your internal team members
Email feasibility packets directly within the CTMS with automated attachments
While a CTMS may offer features that streamline workflows and study operations, it may not be enough to address burnout. To truly ease the work of an overwhelmed research staff, software needs to make the job easier, not harder.
In other words, technology needs to work for people, not the other way around. Site CTMS liberates teams from time spent in frustration over operating and managing the capabilities of their tools, and enables more time caring for patients and conducting research.
Learn more about how Verily Viewpoint Site CTMS can alleviate research team burnout and tech overload for your enterprise research site.
Sources
- What does a modern Clinical Research Coordinator do, and how can technology help?
- 2022 State of Clinical Trial Operations Technology Report
- Re-engineering a Clinical Trial Management System Using Blockchain Technology: System Design, Development, and Case Studies
- Enhancing patient safety and quality of care by improving the usability of electronic health record systems: recommendations from AMIA
- International standards for HCI and usability