FDA Green Lights iRhythm-Verily Zio® Watch and ZEUS System
Long Term iRhythm Partnership Delivers Another Clinically Meaningful Advancement in AFib Diagnosis and Continuous Monitoring
In early 2020, Verily received FDA clearance for our Study Watch wearable device for use in detecting irregular pulses that may indicate a cardiovascular problem. At the time, we shared that we had partnered with iRhythm–a digital health leader focused on cardiac care–to create clinical solutions that could improve cardiovascular care. Two years later, that partnership evolved into the Zio® Watch (Study Watch with Irregular Pulse Monitor) and the ZEUS System, a new precision health solution integrated into clinical care delivery and designed to assist health care providers in identifying and monitoring Atrial Fibrillation (AFib). We are proud to announce that this end-to-end system has now received its own FDA clearance as of July 22, 2022.
By 2030, the CDC estimates that 12 million Americans will have AFib, the most common type of heart arrhythmia. Occurring when the upper atria in the heart beats irregularly, AFib disrupts blood flow, sometimes causing symptoms like fatigue and chest palpitations and significantly increasing an individual’s risk of a stroke. In fact, 1 in 7 strokes are caused by AFib.
AFib is treatable with medicines that can control the heart’s rhythm and that reduce the risk of stroke and with lifestyle changes that mitigate AFib risk factors. But treatment can only happen if a patient and their health care provider are aware of the condition – and as AFib can be episodic, it can be difficult to detect unless an electrocardiogram (ECG) is done while the arrhythmia is present.
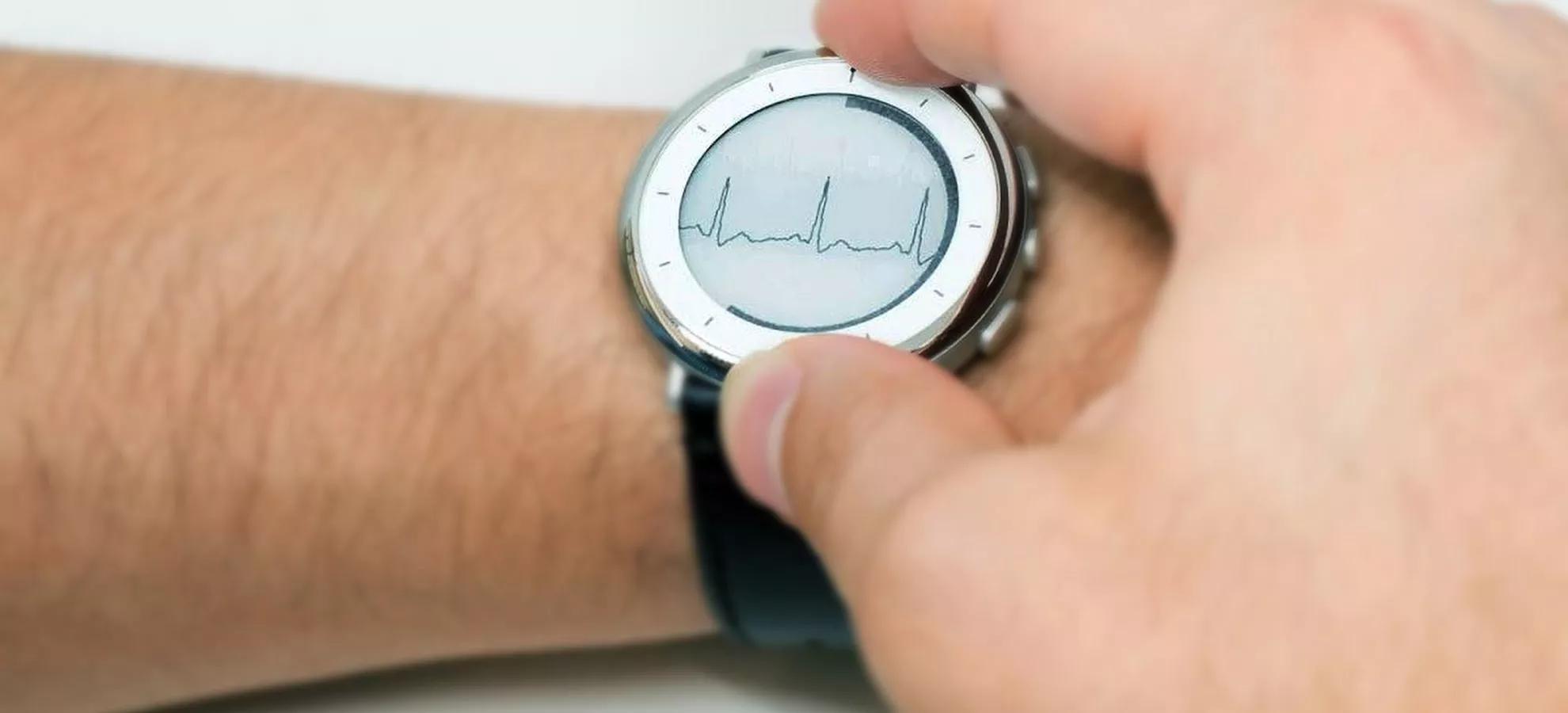
The Zio Watch is a sensor-based device worn on the wrist for non-invasive, clinical grade, long-term continuous monitoring for AFib that began as part of Verily’s Study Watch wearable platform. Study Watch received its own FDA clearance in 2019 and is able to take an on-demand, single lead ECG to help better understand cardiovascular health. The Zio Watch builds on that work evolving to a FDA-cleared prescription-based wrist-worn solution that can detect irregular rhythms indicative of AFib and with the ZEUS System can characterize the amount of AFib over time, thus aiding a clinician in diagnosis. The Zio Watch is differentiated by its ability to integrate with health care providers and deliver the critical data needed to help a physician diagnose the condition in a specific patient and begin treatment.
Working with partners like iRhythm, Verily is putting precision health innovations like Zio Watch in the hands of patients and clinicians, delivering more effective, efficient and personalized care.
Dr. Jessica L. Mega, Chief Medical and Scientific Officer and Co-Founder at Verily.
“Working with partners like iRhythm, Verily is putting precision health innovations like Zio Watch in the hands of patients and clinicians, delivering more effective, efficient and personalized care,” said Dr. Jessica L. Mega, Chief Medical and Scientific Officer and Co-Founder at Verily. “Zio Watch and the ZEUS System fill an important care gap–a medical grade wearable that can help develop precision health interventions for AFib patients, giving the insights needed to help avoid serious clinical events before they occur.”
The Zio Watch works using continuous photoplethysmography (PPG)–measuring light absorption through the skin to determine blood volume–to detect irregularities in the heartbeat. If the watch detects a notable change, a report is sent to their clinician for review. This seamless connection to a healthcare provider goes further than consumer wearables, which may alert a patient of a concerning pattern but then rely on the user’s initiative to seek clinical care.
The Zio Watch is paired with the ZEUS System, which uses sophisticated PPG-based algorithms to detect signs of AFib more accurately, developed using what iRhythm believes to be the world’s largest repository of labeled ECG patient data. The algorithms in the ZEUS System use a novel cloud-based neural network to summarize AFib burden across an extended wear period, delivering clinically meaningful and context-rich reports to clinicians–a significant improvement over reports that simply provide a window into a single moment in time.
Earlier this year, iRhythm presented the Verily Study Watch AF Detection at Home Study at the Heart Rhythm Society annual conference demonstrating that the ZEUS System’s AFib Context Engine (ACE) had high specificity and sensitivity when compared to iRhythm’s Zio patch monitors. Zio Watch will complement the Zio patch monitors to allow patients who require longer-term monitoring to be able to detect, characterize, and manage their AFib in a cost-effective and non-invasive way.
Through projects like Study Watch and partnerships like our work with iRhythm, Verily is taking precision health technologies and learnings and putting them in the hands of physicians. As Zio Watch and the ZEUS System come to market in 2023, people living with or at risk for AFib, as well as their clinicians, will have a new, more convenient and more accurate tool that allows care to be better and more responsive than before.
