Protocol digitization – What exactly is this transformative CTMS feature?
Clinical research sites feel the weight of this ever expanding, ever evolving field that’s imperative for critical medical and science discoveries. Plus, study teams are burdened by many challenges, complexities and tedious, time-consuming tasks, such as executing against PDF, or “paper”, protocols.
To overcome research demands and gridlock, study sites must adopt an advanced clinical trial management system (CTMS), such as Verily Viewpoint Site CTMS.
Site CTMS isn’t your average system. It’s a holistic solution for helping study teams overcome research demands and gridlock with user-friendly features and market-leading innovations, such as protocol digitization.
Protocol digitization is a complex name for a simplifying, AI-driven feature that turns long, complex hard-copied PDF study protocols into intuitive, seamless and accurate digital workflows.
Read on to learn more about this proprietary protocol — and site — transforming feature.
Clinical trial demand far outweighs the workforce supply

What is protocol digitization?
Sites are often bogged down by the daunting task of building, running and analyzing a study as directed by the protocol. And they may be executing these essential tasks by way of a PDF (or “paper”) protocol.
The expertise, time, effort and budget required to convert a PDF protocol into a usable format for trial management is often immense, not to mention creating a mental and morale strain on staff. Compounding all these stressors with increased study volumes, limited workforce and turnover, sites may not realize that it doesn’t have to be this way.
With Site CTMS, there’s a better way to protocol.
With Site CTMS’s proprietary protocol digitization feature that leverages AI, PDF protocols are accurately and efficiently ingested and digested, turning pages and pages of criteria, logic, assessments and data into a digital master study translation. Once digitized, Site CTMS powers and propels clinical and financial workflows for every consented participant.
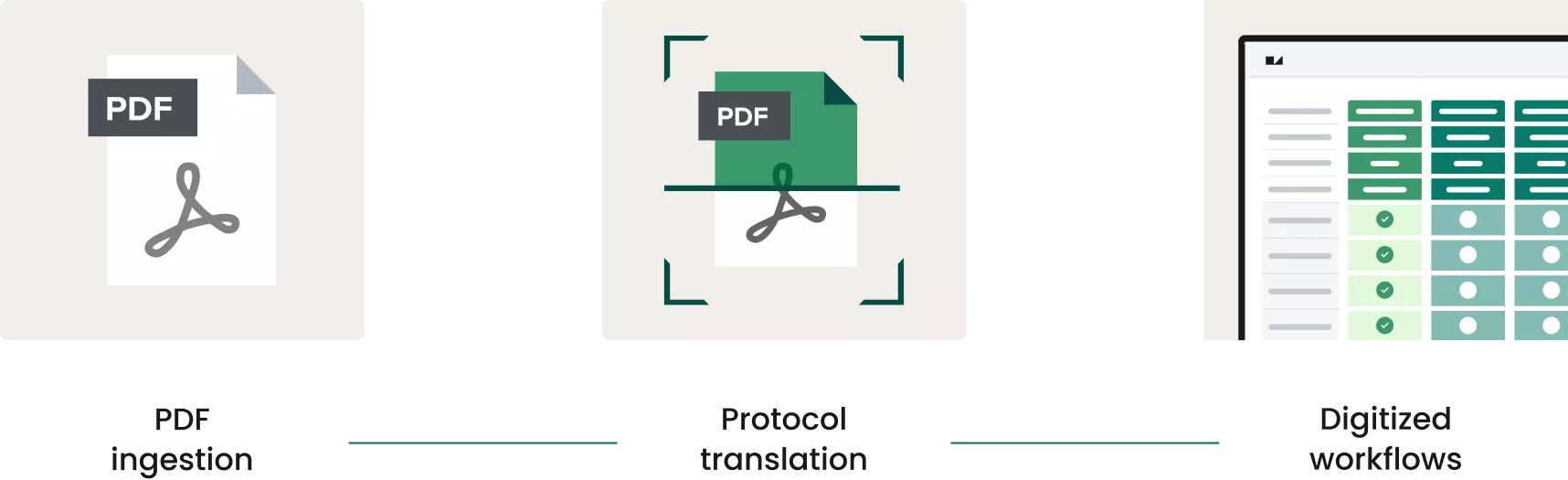
This power also extends beyond managing a study’s schedule of events. With protocol digitization, each study’s schedule of events, inclusion and exclusion criteria, randomization and other critical information are pre-configured and automated within Site CTMS by our tech and teams before a study goes live. In short, it simplifies and it streamlines.
With the ongoing ICH M11 guideline development, Verily has been actively mapping its protocol digitization solution and updating standards to the M11 guideline iterations so that research sites can be provided with the appropriate documentation to demonstrate compliance to this guideline at its official release.
How does protocol digitization work?
The technology- and team-driven process for protocol digitization is a simple, seamless experience for research clients that consists of four basic steps:
- Clients submit their PDF protocol
- Verily Site CTMS AI technology and expert clinical team digitize their protocol
- Verily expert clinical team conducts quality assurance
- Clients review and grant final approval

The seamless experience for research clients is powered by Verily’s deep technical and clinical expertise. A key component of Verily’s protocol digitization technology is its proprietary AI-driven solution, including its ability to accurately deconstruct schedule of assessments (such as study visits and activities tied to those visits), eligibility criteria, and relevant footnotes — including the logic of complex branching, anchoring, cycle iterations, and unplanned visits.
Verily's expert clinical teams, consisting of research coordinators with 7+ years of experience reviewing and digitizing protocols, finalize protocol digitization and conduct extensive quality control to ensure clients receive timely, accurately digitized protocols. With the support of AI, Verily has been able to significantly speed up protocol digitization while maintaining accuracy and quality.
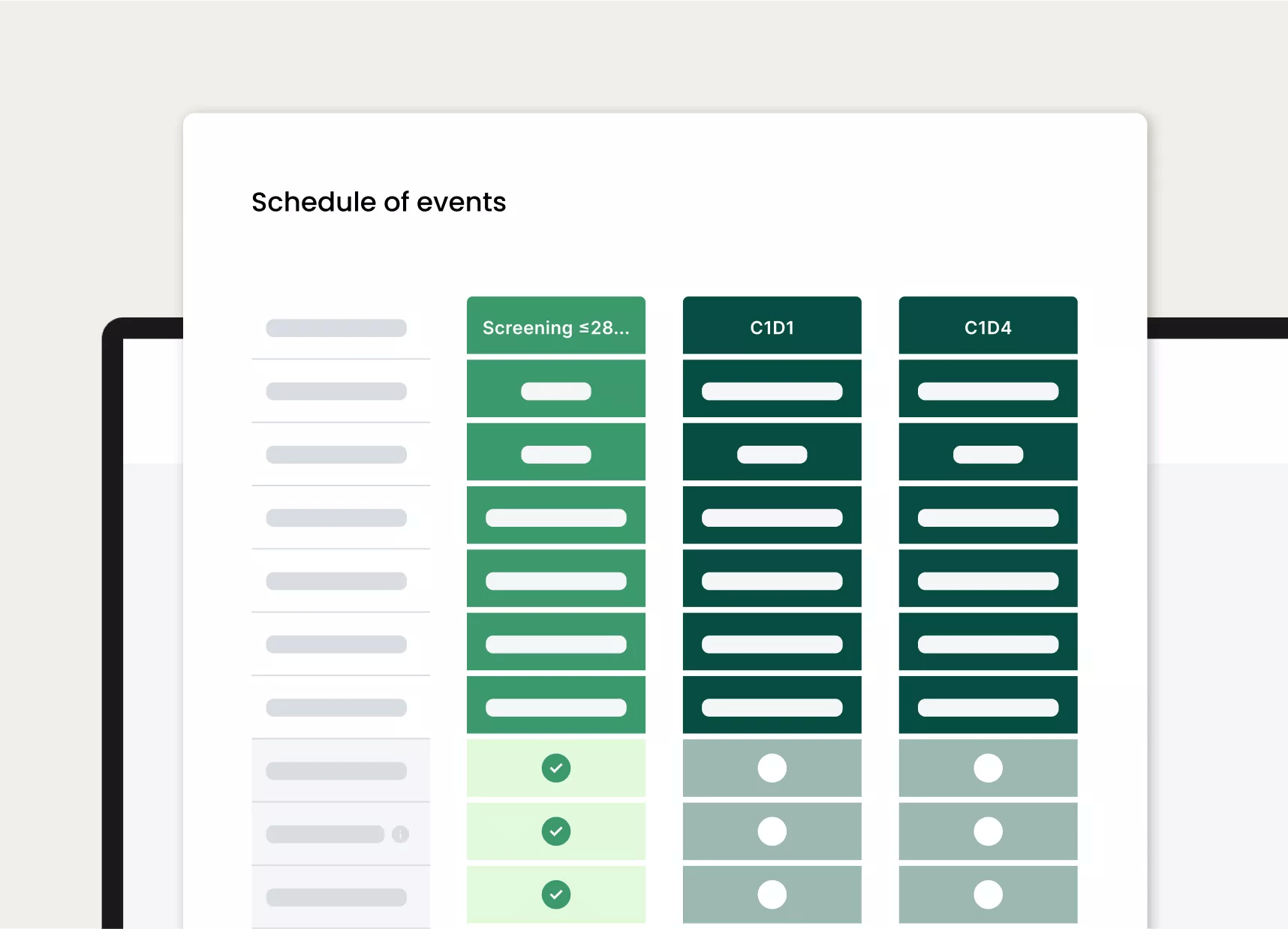
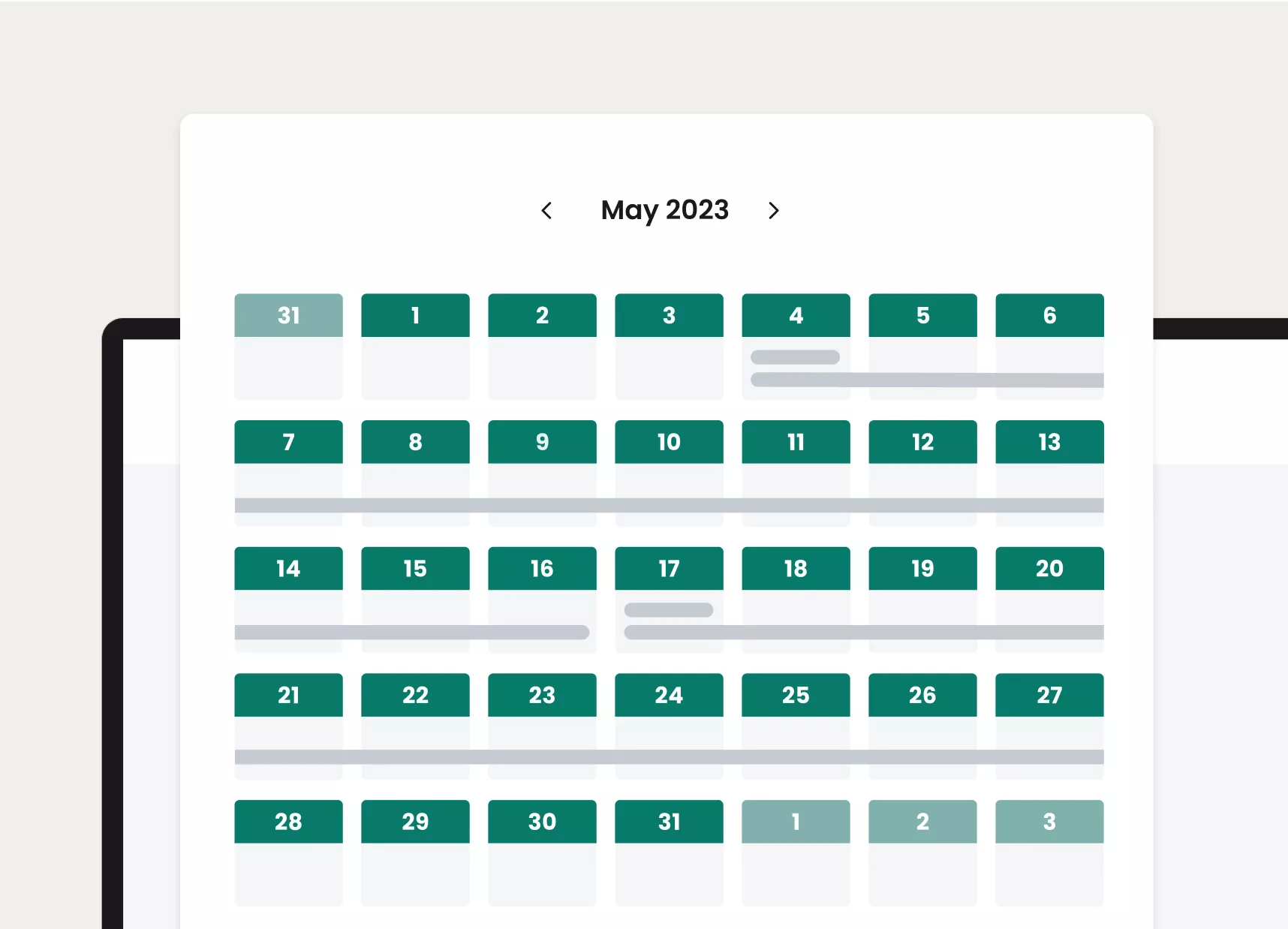
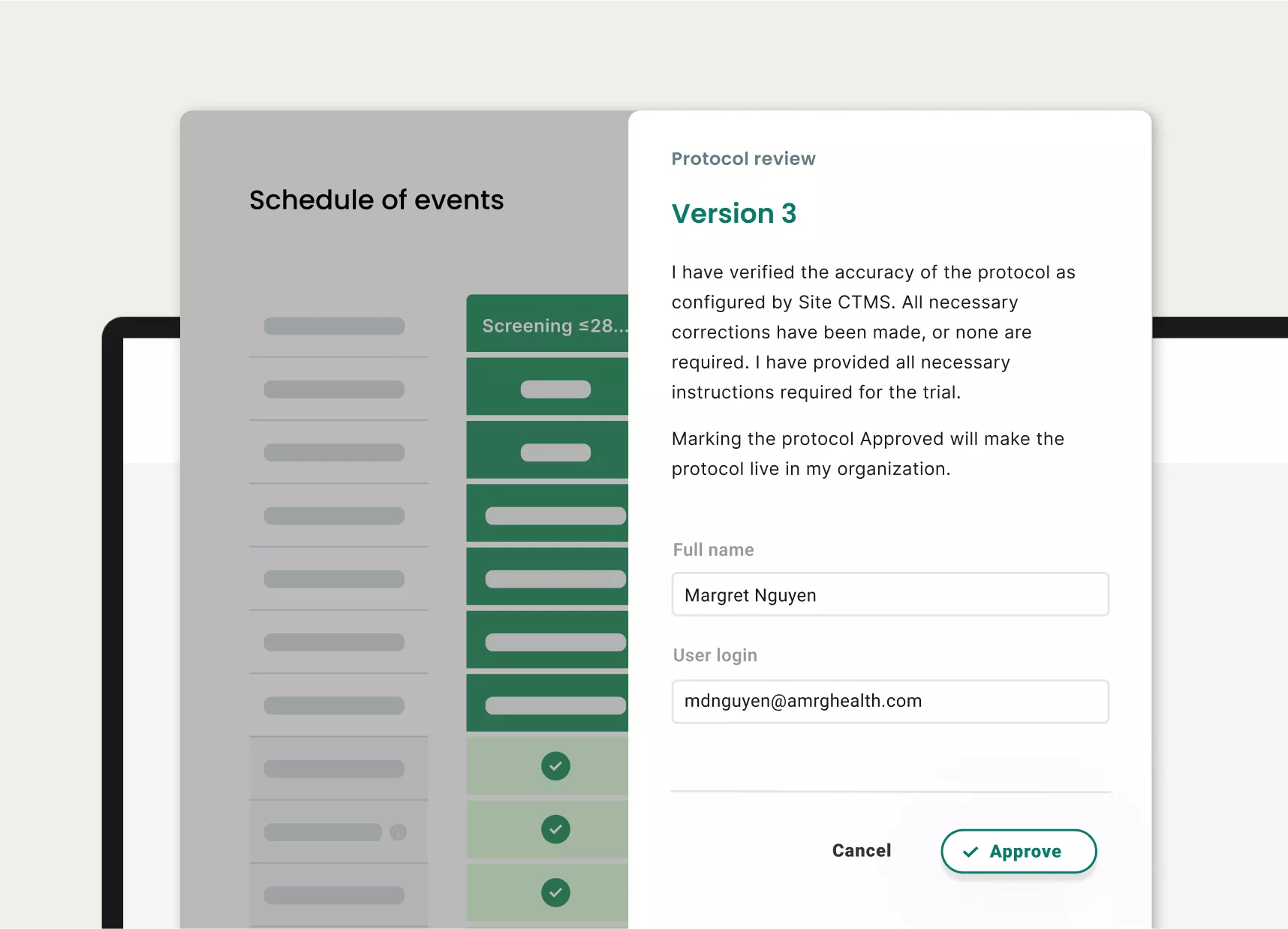
Once digitized, clients can easily view, manage, and adjust studies, including elevated, organized views of activity schedules and more
Immediate detailed schedule of events for participants.
Organized calendar view for each study arm
Optimal version control and revision tracking
How is protocol digitization valuable?
Because protocol digitization is a comprehensive digital translation of a study protocol, its value transcends helping coordinators and clinical staff successfully execute intended procedures and activities, such as providing the pre, during, and post steps to fully facilitate participant visits (although it provides this key feature, too).
Protocol digitization with Site CTMS adds value — from process improvements to research quality to actual monetary value and more — for all study stakeholders, aiding researchers, financial teams and site leadership.
Protocol digitization is also a standard Site CTMS inclusion, which means all clients reap the benefits
Value to researchers
When asked what fuels most researchers, many will say the research itself — the potential to make new discoveries and answer crucial questions. Protocol digitization supports a streamlined, simplified path to discovery that improves research quality and therefore, researcher confidence.
Operational efficiency drivers:
- Can reduce manual tasks for start up and conducting studies
- Can limit reliance on sponsors for tools and resources
- Helps eliminate spreadsheets for workflows and logic
- Fosters lower learning curves for launching and running studies
Quality improvement drivers:
- Supports diminished deviations in managing and performing activities
- May prevent revision and versioning issues per one master translation
- Helps ensure operational continuity, especially with staff changes or PTO
- Auto-population of digital protocols in eBinders via Site CTMS-Florence eBinder integration also helps with quality control
Value to finance teams
Even though sponsors pay study sites to conduct clinical trials and research, budgeting is essential to mitigate financial waste and misuse. However, balancing books isn’t easy.
Through building of a single, easy-to-navigate translation, protocol digitization enables a clearer picture of study coordination costs and its true monetary implications for sites; aligning research and finance teams to more effectively analyze financials and plan budgets.
Learn how a Verily Viewpoint Site CTMS client transformed their research program finances through digitizing protocols and other cost-saving capabilities.
Once translated, budgets are more effectively analyzed and more easily projected
In addition, this enhanced alignment uncovers financial pitfalls, such as revealing areas of under- or over-budgeting, which aids both teams, especially for budget negotiations of future studies. For example, researchers may identify an unmatched budget for the schedule of events, leading to inadequate reimbursement. This learning informs more precise planning to better support the site’s research-related financial competency going forward.
Value to leadership
Site CTMS protocol digitization can improve research productivity, quality, affordability and staff retention. These collective values may ladder up to the leadership level, supporting more productive, harmonized relationships with sponsors and enabling more educated executive decisions, such as taking on additional trials or expanding research capabilities.
Here’s how:
- Staff retention: Taking protocol configuration tasks out of scope can reduce staff burden and repetitive tasks that often lead to turnover.
- Cost containment: Sites limit cost-incurring, additional hires or consultants for manually building workflows into other CTMS software.
- Risks reduction: Centralizing activities and procedures, protocol deviations that may potentiate risks, errors and waste are diminished.
- Ops improvement: Pre-configured workflows facilitate quicker study launches, faster staff onboarding and easier coverage for leave or vacations.
A better way to run trials
As the volume and complexity of research increases, study sites must rely even more on innovative technologies to alleviate pain points that slow down studies, drive away talent, increase costs, threaten output and more. But some tech doesn’t deliver on its promises.
Verily Viewpoint CTMS is an advanced, but intuitive, solution that performs as promised. And it’s the only solution that offers clients the ease and speed of AI-driven digital protocols.
Instead of hiring new employees or burdening existing staff with onerous tasks, like operating against PDF protocols, clients who leverage Site CTMS work smarter, not harder; enhancing workstreams to help meet the growing needs of clinical research and boost job satisfaction.
View protocol digitization in action. Request a guided demo of this transformative feature.
*Annual compounded rate increase, global market
**Annual compounded rate increase, United States market
PTO = Paid time off
Sources
- Bio-IT World Press Release: Clinical Trials Market Size USD 84.43 Billion by 2030.
https://www.bio-itworld.com/pressreleases/2022/07/19/clinical-trials-market-size-usd-84-43-billion-by-2030 - ACRP Special Report: An Assessment of the Adequacy of the Clinical Research Workforce.
https://acrpnet.org/special-report-an-assessment-of-the-adequacy-of-the-clinical-research-workforce




