Viewpoint Study Ops for sponsors
Conduct clinical trials at the speed of digital
Partnering with research sponsors, Verily Viewpoint Study Ops uses proprietary technology, such AI-driven Viewpoint Digital Protocols, to help organizations seamlessly transition to digital-centric trial operations, driving efficiencies and cost-savings.
Note: Some features are under development.

Digital Protocols
Drive and execute efficient clinical trials with a digital study blueprint
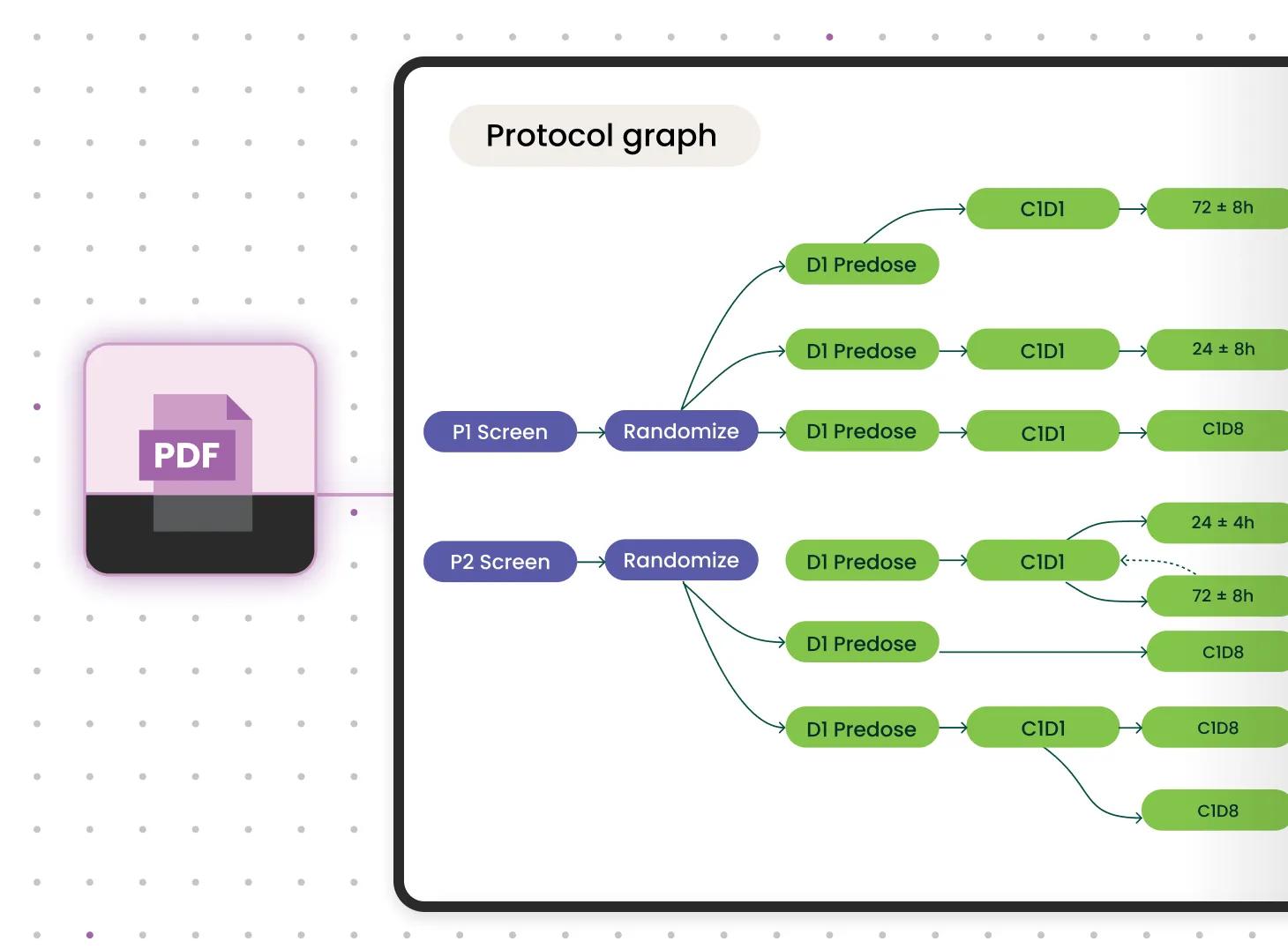
Refined over 8+ years, Verily’s proprietary, AI-driven digital protocol solution aims to help sponsors design and run faster, less costly trial operations by quickly capturing nuanced study details from static PDF protocols — the schedule of assessments, I/E criteria, footnotes, and more — to create a dynamic, digital data model in relevant data standards for interoperability.
Executed with AI technology, guided by research experts
Explore our webinar: Unlocking efficiency with Digital Protocols
Digital Protocols transforms PDF protocols into Verily’s proprietary digital format in a two-step process: AI automates the extraction of most relevant elements, and a team of 50+ clinical experts finalizes and performs quality checks on the output. Verily has accelerated the already optimizing process with AI, fine-tuning large language models (LLM) like PaLM-2.
Learn more with an on-demand webinar: Accelerate Clinical Trials - Unlocking Efficiency with Digital Protocols
Efficiency-driving benefits - Some
features are under development
AI-driven insights from over 16,000 protocol versions can help improve trial design, by predicting and mitigating risks related to protocol complexity, enrollment, and timelines.