From data gaps to continuous insight — A persistent evidence engine that reflects real life
For decades, the bedrock of biomedical breakthroughs has been rigorous, site-based clinical research. This established model has served as the vital evidence engine for progress, shaping our understanding of human health. Its methodical approach to clinical trials and controlled studies has long set the gold standard for generating robust evidence.
However, as the healthcare landscape continues its rapid evolution, this established model faces challenges. The very nature of traditional research, while scientifically sound, can inherently limit its scale, speed, and inclusivity. As Sarah Short, MPH, a Verily biostatistician with extensive experience in late-phase and observational studies, explains, “The traditional research model often limits us to collecting information in snapshots during site visits, leaving the continuous story of a person's day-to-day health largely invisible. This approach has also required participants to live near major research hospitals and travel for visits. That logistical burden not only restricts who can participate, but it can also cause people to disengage from studies, creating even more gaps in the data and potentially impacting the generalizability of the research.”
The result is research findings that, while invaluable, often demonstrate efficacy in a trial but fail to predict real-world effectiveness. Traditional clinical trials are limited in two fundamental ways:
- They only collect specific, predefined, snapshots of data. This means researchers may miss crucial information about a person’s complete health journey.
- They study a narrow segment of the population that can access the trial.
Because of these limitations, traditional clinical trial findings may not accurately predict how effective a treatment will be in the real world with a diverse population. Traditional data might show what happened, like a patient switching therapies, but it leaves the crucial context — the ‘why,’ such as intolerable side effects — completely out of sight. To advance medicine that's clinically-relevant and more complete, we need an evolution in evidence generation.
We can’t overemphasize the power of and need for collecting rich, diverse contextual data types from outside the health system, such as wearable data, genetics data, and frequent participant survey responses. Too often, this data is largely invisible.
Andrew Trister, MD, PhD, Verily Chief Medical and Scientific Officer
A key step, but not the final destination: The rise of DCTs
Recognizing these limitations, the clinical research industry took important steps forward. Decentralized clinical trials (DCTs) emerged as a crucial innovation, leveraging technology to bring research directly to patients' homes. This was a significant move, reducing geographic barriers and making participation more accessible. Verily’s experience as sponsor of the Baseline Health Study demonstrated an ability to keep participants highly engaged over the long term in studies with decentralized components, retaining 94% of participants over four years.
Yet, even with the benefits of decentralization, many DCTs remain focused on a specific period of time. They often digitize the traditional clinical trial mindset, offering intense and truncated engagements where participation is limited to a short period before it stops completely. This approach doesn’t mirror people’s real-world daily experiences of disease. As Andrew Trister, MD, PhD and Verily's Chief Medical and Scientific Officer, points out, “This contrasts sharply with the vision of a continuous, long-term view of a person, which is essential for truly understanding health. To holistically transform evidence generation, we must move beyond merely decentralizing trials to building a decentralized, persistent evidence engine with continuous participant engagement.”
A Verily expert-authored article recently-published in Clinical Therapeutics emphasizes that elements of DCTs may excel at widening participant reach, but they need to be thoughtfully and intentionally applied to make meaningful transformations in how clinical research is conducted. To build a more complete and clinically meaningful picture, the research community must move toward research models that generate richer, longitudinal insights for representative populations.
The shift to a persistent evidence engine
This paradigm shift represents a fundamental evolution: from episodic studies tied to physical research sites to a continuous, participant-centric engine that can engage people wherever they are. Viewpoint Evidence powers this transition, allowing researchers to move beyond temporary cohorts and build a lasting community of engaged, re-contactable participants. This unique ability to re-contact participants transforms real-world data (RWD) from static snapshots into dynamic, ongoing conversations. It creates a persistent evidence engine, enabling you to rapidly investigate safety signals or gather evidence for new hypotheses without the cost and delay of launching new studies.
A key application is bringing research sponsors’ existing trial participants into the participant community alongside Verily's pre-engaged participants, which allows Verily to link traditional study data capture with medical record history and wearable data, conduct ePROs, and more. As Sarah Short explains, “Amassing a large number of people in such a program creates an asset that exponentially grows in value over time. This commitment to a continuous, evolving data asset supports the practical ‘how-to’ of RWD implementation by providing a clear, architectural model for the future of producing biomedical evidence. This will be a new framework that provides unique, transformative capabilities that change how we understand health.”
Comprehensive, multimodal data unlocks precision
Longitudinality and integrated data
Viewpoint Evidence, built on the Verily precision health platform, allows biomedical researchers to natively integrate diverse data types, providing a far richer view of health than medical records alone. According to Dr. Trister, “We can’t overemphasize the power of and need for collecting rich, diverse contextual data types from outside the health system, such as wearable data, genetics data, and frequent survey responses. Too often, this data is largely invisible.”
The true challenge, however, is turning this mix of complex information into clear, actionable evidence. This is where Verily's AI-native precision health platform provides the solution, supporting the scalable integration of most healthcare datasets, and using advanced tools to enrich and unify them in Verily’s proprietary clinical data model. In addition, AI unlocks critical insights buried in unstructured data like clinician notes, creating clean, bespoke variables to answer your most specific questions — such as uncovering the precise reason a patient switched their medication.
Verily’s ability to continually collect and integrate diverse data, which pharma companies simply can't source from EHRs and claims data alone, creates a more complete evidence base. This helps our clients accelerate research and design smarter studies from the start.
Lisa Lehmann, MD, PhD, Verily Medical Director for Clinical Research
Unprecedented research agility
Verily’s platform design also allows for unprecedented research agility. “Verily’s ability to quickly assemble different kinds of cohorts from the larger engaged participant base means if a pharmaceutical company needs to answer an emerging question, such as understanding specific side effects from a drug, our platform can facilitate rapid deployment of a custom survey to relevant participants,” explains Dr. Trister.
Along with surveys, this can help accelerate the time-to-insight for urgent questions that clarify existing data points, enabling research sponsors to offer participants follow-up testing, or run entirely new studies using novel designs, such as pragmatic trials that take place in settings where everyday care happens to increase convenience of participation. This combination of speed and nimbleness can meaningfully accelerate pharmaceutical R&D, helping teams move from discovery to decision with greater efficiency, facilitating a larger return on investment.
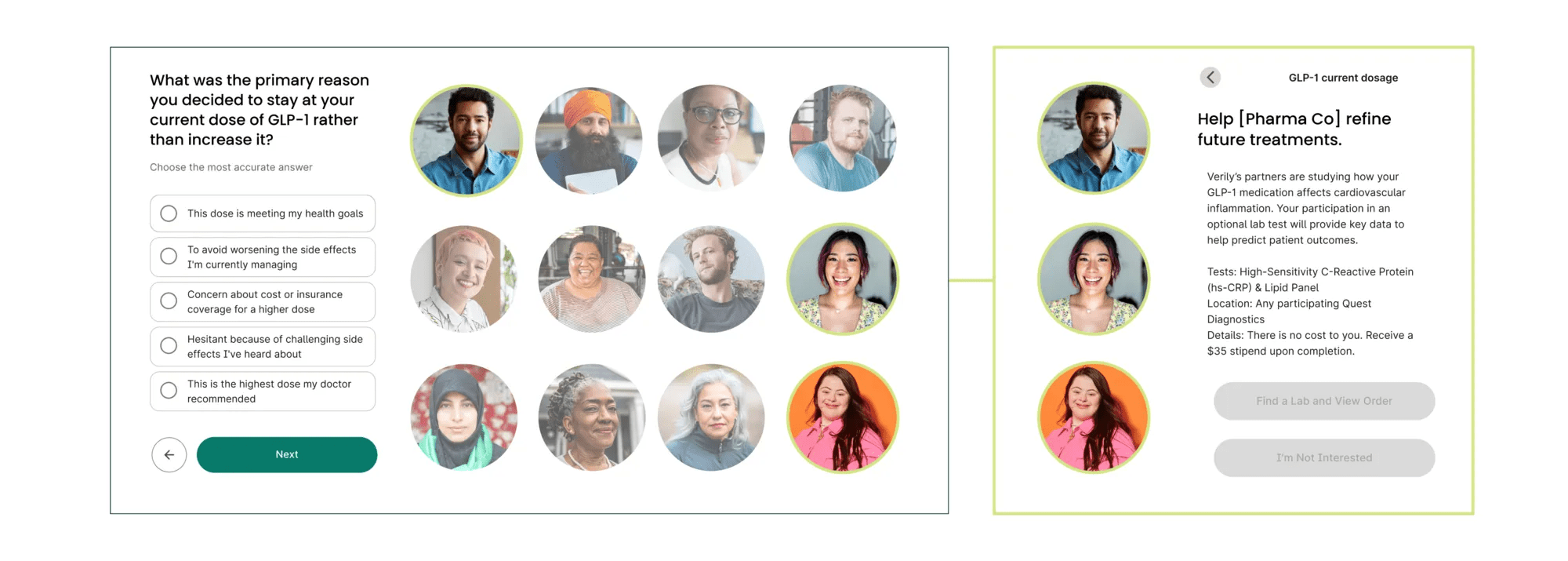
Verily Viewpoint Evidence enables pharma companies to easily identify people on a specific drug, and quickly send them a survey to ask context driven questions to understand patient experience.
More comprehensive data = more actionable insights
The benefits of a new engine with continuous evidence generation are clear. By moving beyond siloed data and episodic studies, we will begin to understand the complete patient journey with unprecedented clarity. This comprehensive, longitudinal view allows us to better predict individual health trajectories, identify variations, and understand the impact of real-world interventions.
By building this deep, continuous, and representative view of individuals — one made possible by removing the logistical barriers of traditional research — the biomedical community can gain the contextual understanding necessary to truly personalize medicine — actual precision health. This is a journey towards a future where health insights are not just generated, but are continuously delivered, understood, and acted upon, fostering a tighter bond between research and care for every individual.
Up next: Unveiling more evidence advancements
Stay tuned for the next post that will reveal — and connect — the innovative pieces that enable Verily’s new evidence-generation approach — from engaging and empowering consumer health experiences that gather day-to-day signals, to a new registry designed to create an even larger, re-contactable participant base, and beyond. We’re seizing the opportunity to improve RWD quality by filling data gaps, expanding the reach of research, and closing the research-to-care loop.
Until then, take a closer look at the 4-year long, Verily-led Baseline Health Study, which is a cornerstone of our real-world evidence capabilities that resulted in a vast, deeply phenotyped dataset, and a large cohort of participants.


