Site CTMS
Alleviate research gridlock and burnout with advanced, user-friendly technology
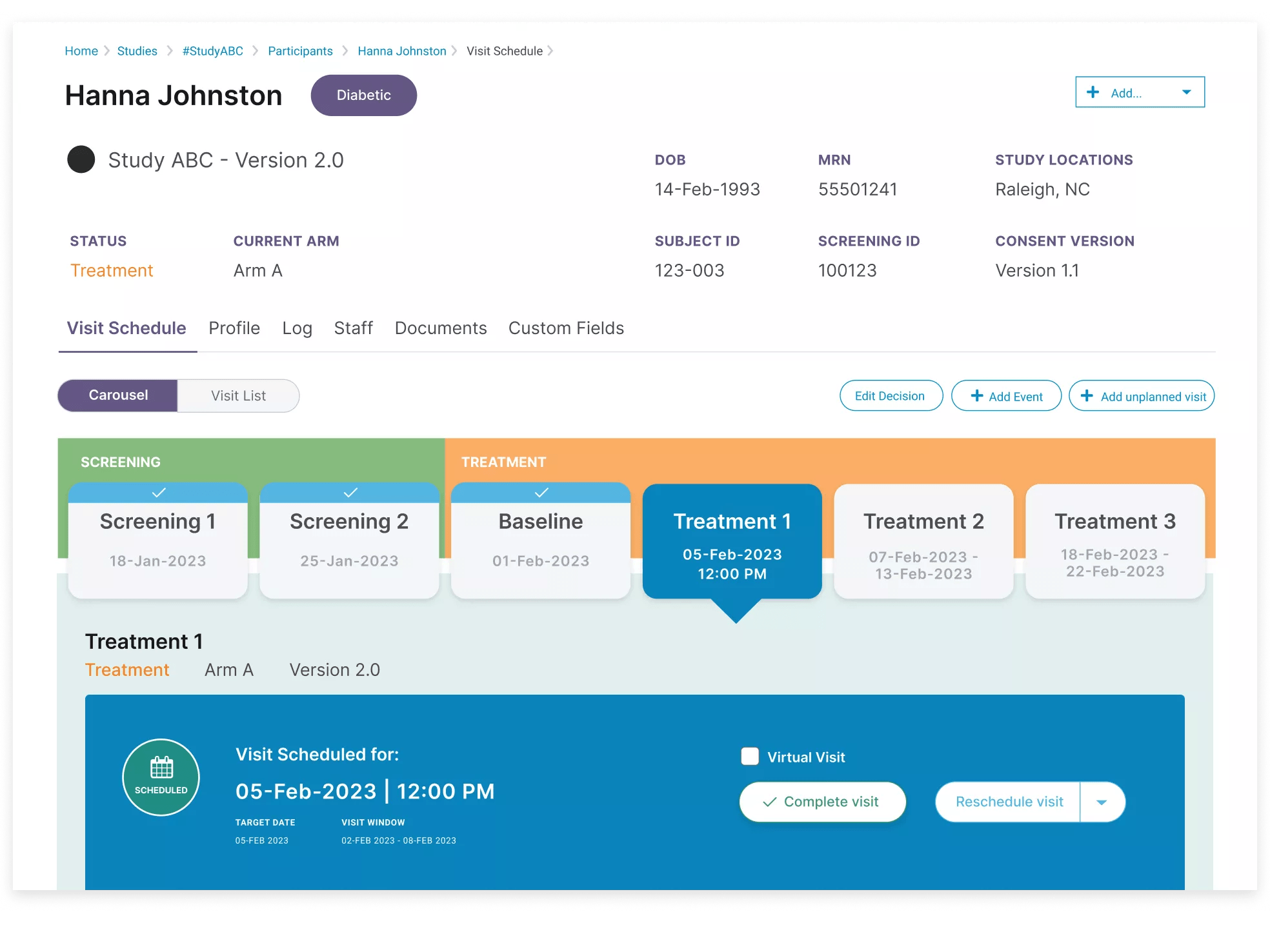
A state-of-the-art clinical trial management system (CTMS), built for enterprise research sites, digitizes study protocols and eliminates siloed manual processes to enable more efficient research, higher-quality output and maximum research revenue.
Simplify study workflows
At Verily, user experience is everything. Easy-to-use by all staff, Verily Viewpoint Site CTMS was built for researchers, by researchers, helping eliminate time spent on learning new systems, fostering employee satisfaction and replacing tedious software and tasks.
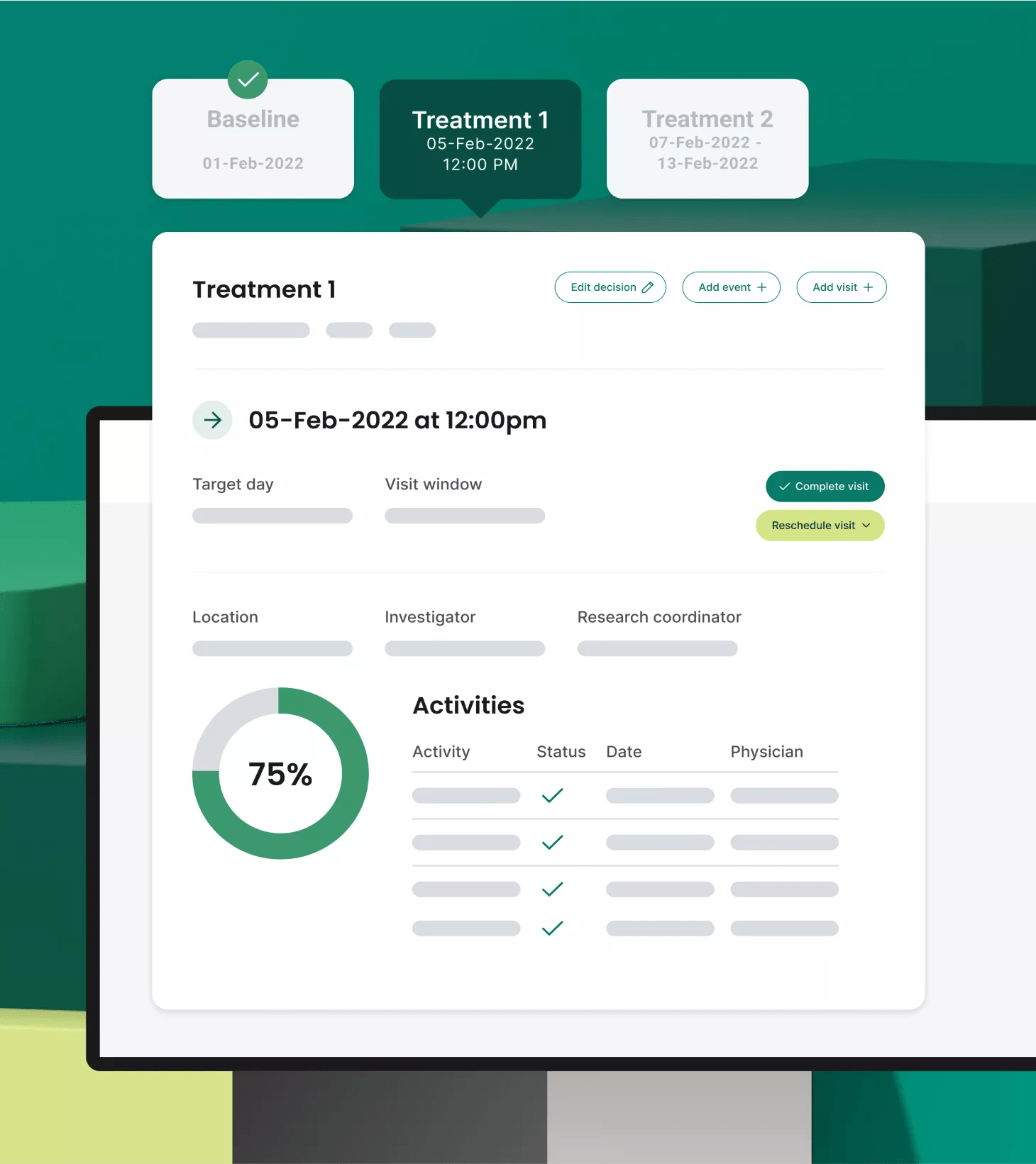
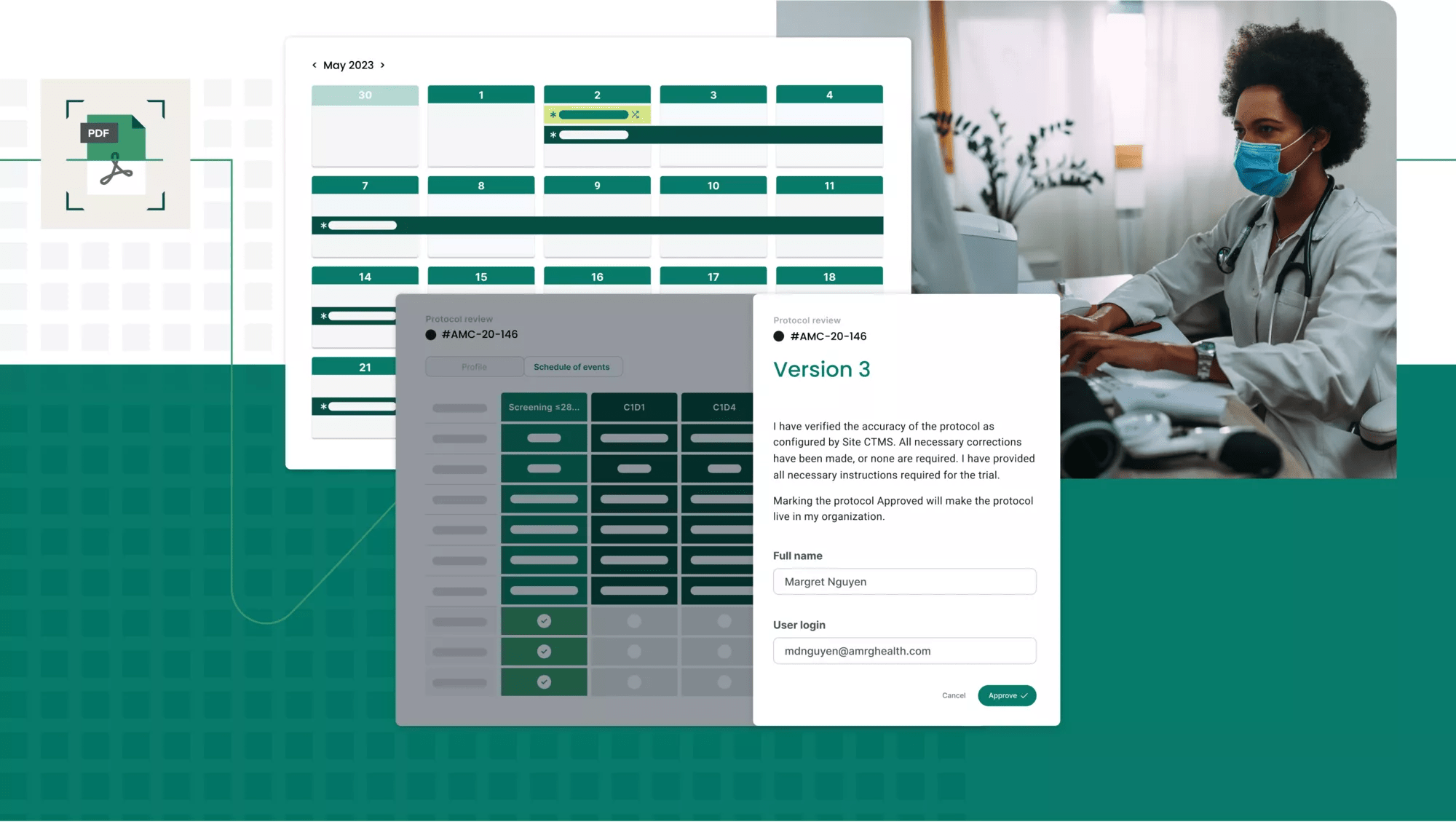
Protocol digitization
Work smarter, not harder
Through our proprietary protocol digitization function, PDF protocols are accurately and efficiently ingested and digested, configuring pages and pages of criteria, logic, steps and data into a digital master study translation, powering all clinical and financial workflows for every consented participant.

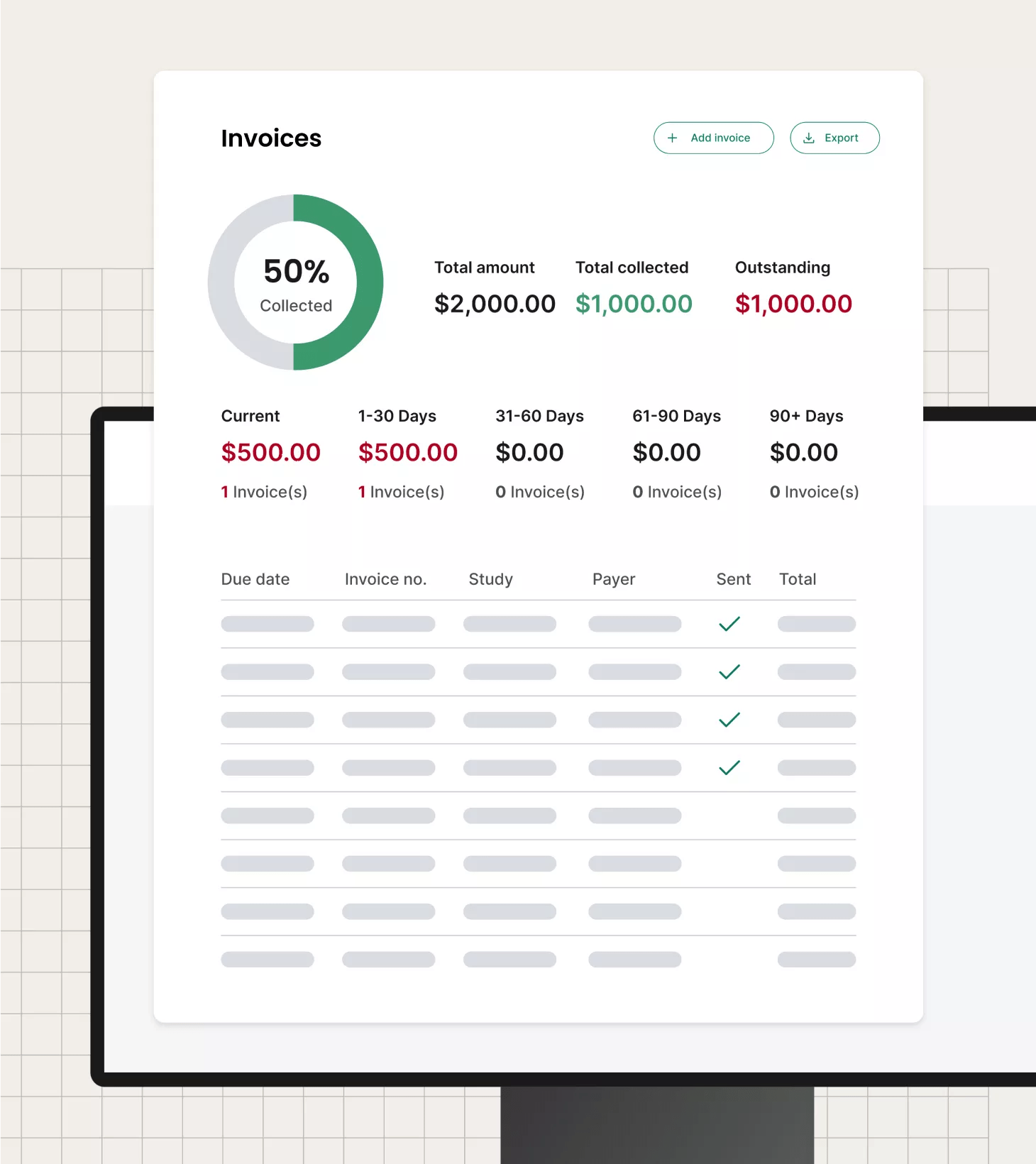
Maximize research revenue
View financials presented in sophisticated, but clear, dashboards to quickly analyze revenue, costs and profitability. Through these advanced data capture and analytics tools, precisely view resource and payroll costs, find reimbursement issues or waste and more.
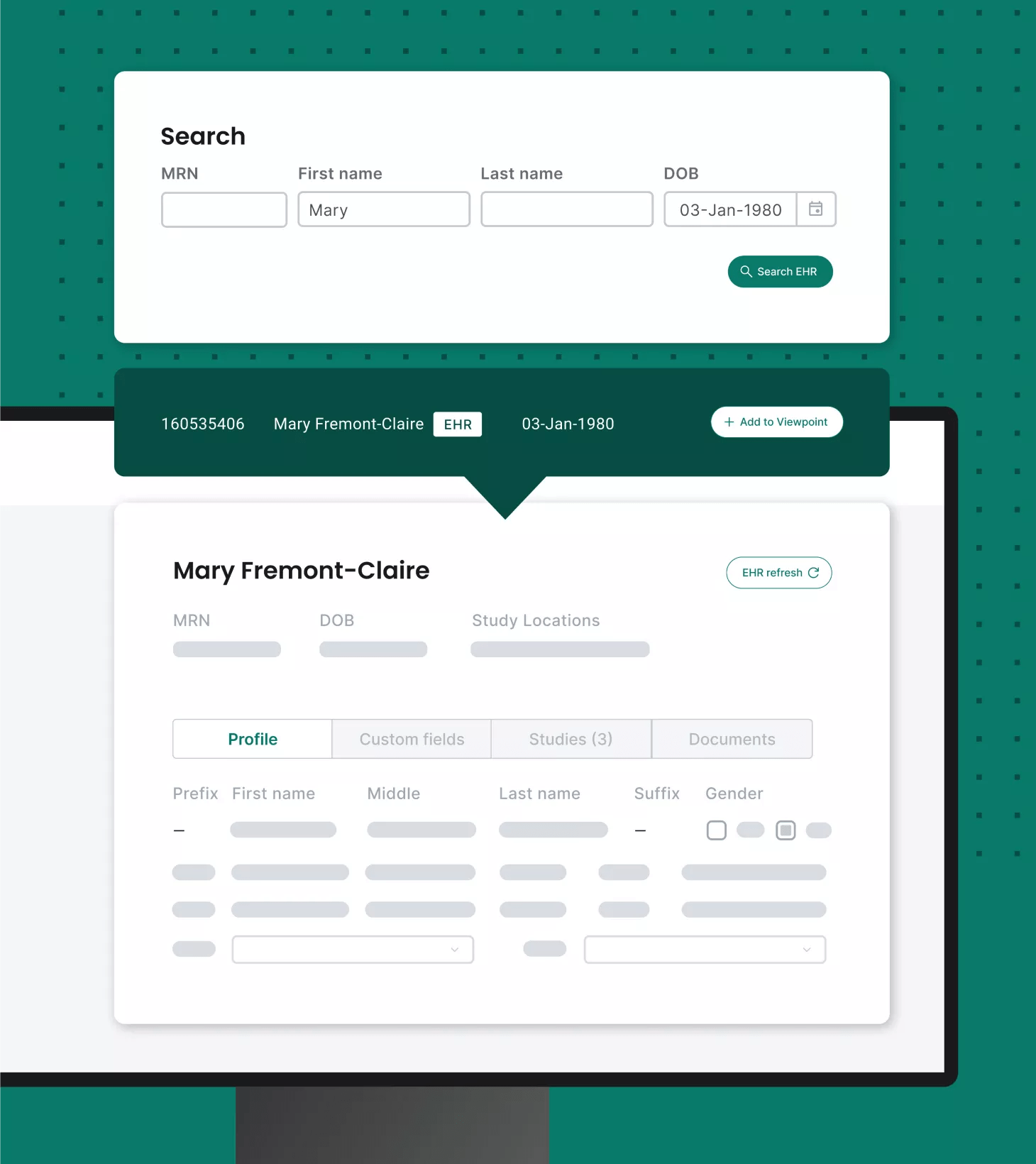
Integrate and unify systems
Site CTMS offers integrations across eClinical solutions, such as EHRs, eRegulatory binders, payments and more.
Support all site stakeholders
Set your site free
Free your staff to focus on research. And enable more friction-free research paths with next-gen CTMS features that support quality output and therefore, researcher confidence, by cutting manual tasks and offering a one-stop-shop for all activities.
Set your site free
Free your staff to focus on research. And enable more friction-free research paths with next-gen CTMS features that support quality output and therefore, researcher confidence, by cutting manual tasks and offering a one-stop-shop for all activities.
